530263
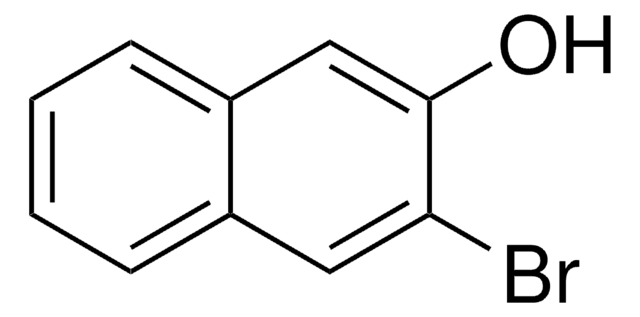
6-Cyano-2-naphthol
97%
Synonym(s):
2-Cyano-6-hydroxynaphthalene, 2-Cyano-6-naphthol, 2-Hydroxy-6-naphthonitrile, 6-Cyano-2-hydroxynaphthalene, 6-Hydroxy-2-naphthalenecarbonitrile, 6-Hydroxy-2-naphthonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NCC10H6OH
CAS Number:
Molecular Weight:
169.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
165.5-170.5 °C (lit.)
functional group
nitrile
SMILES string
Oc1ccc2cc(ccc2c1)C#N
InChI
1S/C11H7NO/c12-7-8-1-2-10-6-11(13)4-3-9(10)5-8/h1-6,13H
InChI key
WKTNIBWKHNIPQR-UHFFFAOYSA-N
General description
6-Cyano-2-naphthol (6CN2) is an aromatic alcohol that can be synthesized from 6-bromo-2-naphthol. It is a superphotoacid with the ground state pKa* value of 8.4 and excited state pKavalue of 0.2, respectively. 6CN2 protonates PANI-ES (polyaniline emeraldine salt) to form PANI-EB (emeraldine base), which shows enhanced conductivity. The proton-transfer kinetics and photophysical behavior of 6CN2 have been investigated.
Application
6-Cyano-2-naphthol (6-Hydroxy-2-naphthonitrile, 2-cyano-6-naphthol) may be used in the preparation of:
- 5-bromo-6-hydroxy-2-naphthonitrile
- 5,7-dibromo-6-hydroxy-2-naphthonitrile
- 5-chloro-6-hydroxy-2-naphthonitrile
- 6-(2-imidazolyl)-2-naphthol
- dodecaethylene glycol di-6-cyano-2-naphthyl ether
- 6-cyano-2-naphthyl trifluoremethanesufonate
- 2-(6-cyano-naphthyl)2,3,4-tri-O-acetyl-β-D-xylopyranoside
- 1,5-bis(7-amidino-2-naphthalenoxy)-3-oxapentane dihydrochloride
Reactant for:
- Palladium-catalyzed reduction
- Nickel-catalyzed cross-coupling reactions
- Palladium-catalyzed Heck reactions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anna Siegbahn et al.
Organic & biomolecular chemistry, 13(11), 3351-3362 (2015-02-07)
Proteoglycans (PGs) are macromolecules that consist of long linear polysaccharides, glycosaminoglycan (GAG) chains, covalently attached to a core protein by the carbohydrate xylose. The biosynthesis of GAG chains is initiated by xylosylation of the core protein followed by galactosylation by
Doping of Polyaniline with 6-Cyano-2-naphthol.
Das D, et al.
The Journal of Physical Chemistry B, 118(45), 12993-13001 (2014)
Ultrafast excited-state proton transfer from cyano-substituted 2-naphthols.
The Journal of Physical Chemistry A, 101(25), 4602-4605 (1997)
ssDNA templated assembly of oligonucleotides and bivalent naphthalene guests.
Janssen PGA, et al.
Soft Matter, 6(7), 1494-1502 (2010)
T Nakayama et al.
Chemical & pharmaceutical bulletin, 41(1), 117-125 (1993-01-01)
By developing 6-amidino-2-naphthyl 4-guanidinobenzoate (I, FUT-175) as a basic structure, its various derivatives were synthesized and their inhibitory activities on trypsin, plasmin, kallikrein, thrombin, C1r and C1s as well as on complement-mediated hemolysis were examined. The protective effect of these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service