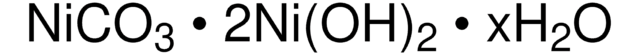377449
Manganese(II) carbonate
≥99.9% trace metals basis
Synonym(s):
Manganese carbonate, Manganese(2+) carbonate, Manganous carbonate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
MnCO3
CAS Number:
Molecular Weight:
114.95
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Assay:
≥99.9% trace metals basis
form:
powder
solubility:
dilute aqueous acid: slightly soluble(lit.)
Recommended Products
Quality Level
Assay
≥99.9% trace metals basis
form
powder
impurities
≤1,000.0 ppm Trace Metal Analysis
mp
>200 °C (lit.)
solubility
dilute aqueous acid: slightly soluble(lit.)
density
3.12 g/mL at 25 °C (lit.)
SMILES string
[Mn++].[O-]C([O-])=O
InChI
1S/CH2O3.Mn/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
XMWCXZJXESXBBY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Manganese(II) carbonate is a chemical compound that has a structure similar to calcite, with octahedral co-ordination symmetry. It is a carbonate that is insoluble in water and on treatment with acid it gives water soluble salts. It is a widely used material in plant fertilization as an additive that cures the magnesium deficiency in crops.
Application
Manganese(II) carbonate can be used as:
- A primary electrode material in asymmetric supercapacitors for improving the charge storage capacity and overall performance of the supercapacitors.
- A precursor for synthesizing manganese oxide, which is used as a component in various electrochemical applications, including batteries and supercapacitors.
- A precursor material in the synthesis of oxygen vacancy-rich nitrogen-doped manganese carbonate (MnCO2@N) microspheres. These microspheres are then employed as cathode materials in aqueous zinc-ion batteries (ZIBs) to enhance their electrochemical performance.
- A potential electrocatalyst for the oxygen evolution reaction (OER) in water splitting applications.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Maciejewska et al.
Nanomaterials (Basel, Switzerland), 10(3) (2020-03-04)
Herein, a novel synthesis method of colloidal GdPO4:Mn2+,Eu3+ nanoparticles for luminescent nanothermometry is proposed. XRD, TEM, DLS, and zeta potential measurements confirmed the crystallographic purity and reproducible morphology of the obtained nanoparticles. The spectroscopic properties of GdPO4:Mn2+,Eu3+ with different amounts
Structures of hydrothermally synthesized cobalt (II) carbonate and nickel (II) carbonate
Pertlik F
Acta Crystallographica Section E: Crystallographic Communications, 42(1), 4-5 (1986)
Jyoti Patel et al.
Nanomaterials (Basel, Switzerland), 10(5) (2020-05-24)
Norfloxacin (NOFX), a broadly used fluoroquinolone antibiotic, has been a subject of great concern in the past few years due to its undesirable effect on human beings and aquatic ecosystems. In this study, novel Mn doped ZnS (Mn:ZnS) quantum dots
Manganese (II) salts as efficient catalysts for chemo selective transesterification of beta-keto esters under non-conventional conditions
Krishnaiah G, et al.
Tetrahedron Letters, 54(7), 703-706 (2013)
Manganese compounds
Reidies AH
Ullmann's Encyclopedia of Industrial Chemistry, 42(1), 4-5 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service