Macmillan Imidazolidinone Organocatalysts
Metal-free Asymmetric Catalysis
Developed by Professor David MacMillan at Caltech, imidazolidinone-based OrganoCatalysts are designed to serve as general catalysts for a variety of asymmetric transformations. The first highly enantioselective organocatalytic Diels-Alder reaction using (5S)-2,2,3-trimethyl-5-phenylmethyl-4-imidazolidinone monohydrochloride was reported by MacMillan in his pioneering work in 2000 (Scheme 1).1 The activated iminium ion, formed through condensation of the imidazolidinone and an α,β-unsaturated aldehyde, underwent reaction with various dienes to yield [4+2]-cycloadducts in excellent yields and enantioselectivities.
![Enantioselective Organocatalytic Diels-Alder Reaction Using (5S)-2,2,3-Trimethyl-5-Phenylmethyl-4-Imidazolidinone Monohydrochloride Reaction of the activated iminium ion, formed through condensation of the imidazolidinone and an α,β-unsaturated aldehyde, with various dienes to yield [4+2]-cycloadducts in excellent yields and enantioselectivities.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/chemistry-and-synthesis/reaction-design-and-optimization/enantioselective-organocatalytic/enantioselective-organocatalytic.jpg)
Scheme 1
Other organocatalytic transformations such as 1,3-dipolar cycloadditions,2 Friedel-Crafts alkylations,3 α-chlorinations,3 α-fluorinations,4 and intramolecular Michael reactions5 were reported using MacMillan’s Imidazolidinone OrganoCatalysts, all proceeding with high levels of enantioselectivity (Scheme 2).

Scheme 2
MacMillan found an optimized structure in (2S,5S)-(−)-2-tert-butyl-3-methyl-5-benzyl-4-imidazolidinone for the Friedel-Crafts alkylation of indoles (Scheme 3). 5,6
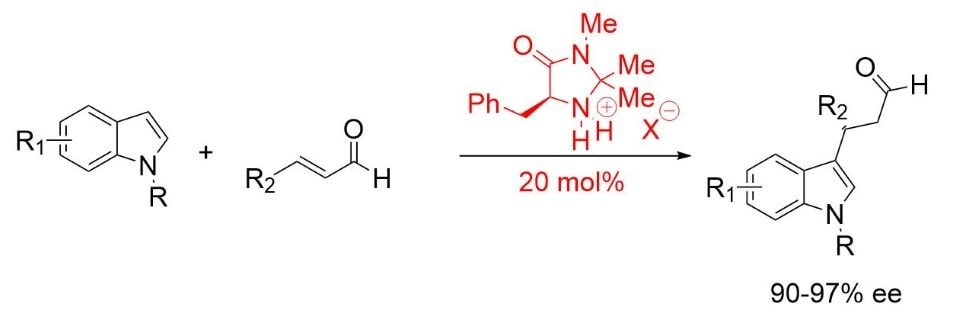
Scheme 3
The synthetic utility of this concept was later demonstrated in the total synthesis of (–)-flustramine B, a biologically active pyrroloindoline-containing alkaloid (Scheme 4).7
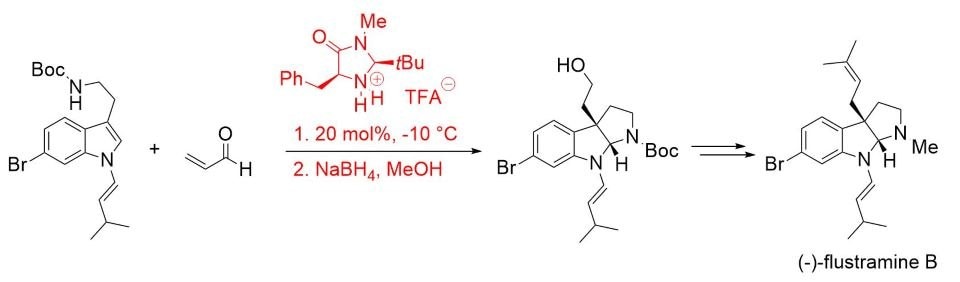
Scheme 4
More recently, a photochemical enantioselective α-alkylation protocol has been developed and applied to the total synthesis of (−)-enterolactone and (−)-enterodiol. The reaction proceeds in the presence of visible light and in the absence of a photocatalyst via a light-activated charge-transfer complex. (Scheme 5).8
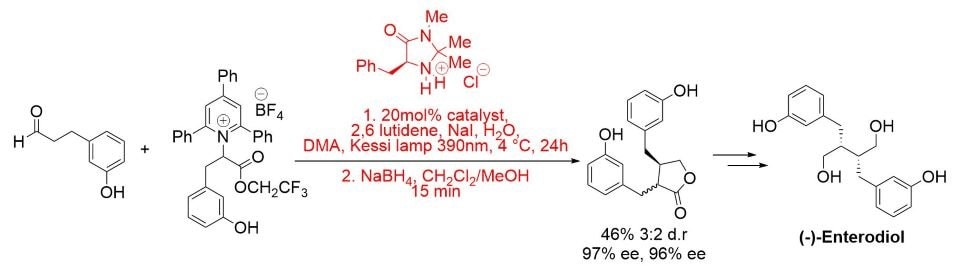
Scheme 5
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?