Primary Human Spheroid-Qualified Hepatocyte Culture Protocol
- 3D Hepatocyte Spheroids
- Spheroid-Qualified Hepatocyte Culture Materials
- Thawing and Plating Spheroid-Qualified Hepatocytes for 3D Spheroid Formation
- 3D Hepatocyte Spheroid Culture Results
3D Hepatocyte Spheroids
Our portfolio of plateable Hepatocytes includes spheroid-qualified Hepatocytes for 3D cell culture applications. While our plateable and suspension Hepatocytes are ideal for 2D cell culture applications, our spheroid-qualified plateable Hepatocytes can provide more relevant models for applications such as enzyme induction, toxicity, drug screening, transporter efflux activity, potential drug-drug interactions, and disease modeling.
Our collection of spheroid-qualified plateable Hepatocyte is isolated from whole human livers and consists of a homogenous population of cells, with each donor providing documented consent for research use of non-transplantable organs or tissues. Every lot is guaranteed to contain a minimum of 4 million viable cells per vial, maintaining a post-thaw viability of ≥70%. These Hepatocytes undergo extensive characterization, encompassing mRNA induction data and enzyme profiling data. Additionally, they are validated to form 3D spheroids when cultured for a minimum of 7 days. In this protocol, we demonstrate how to thaw, plate, and form Hepatocyte spheroids from human spheroid-qualified plateable Hepatocytes.
Spheroid-Qualified Hepatocyte Culture Materials
- Spheroid Qualified Hepatocytes
Note: Upon receipt, immediately store cryovial(s) in vapor phase liquid nitrogen. - Ultra-Low Attachment Multiple Well Plate
- Spheroid Maintenance Medium:
Note: For Dexamethasone, dissolve 25mg into 32 mL of ethanol (100%) to make 2mM stock. For Linoleic acid, dissolve 100mg into 2mL of ethanol (100%) to make 50mg/mL stock. Once prepared media is stable for at least 4 weeks at 4°C.
Thawing and Plating Spheroid-Qualified Hepatocytes for 3D Spheroid Formation
This protocol was performed in a Class II laminar flow biohood and using an incubator set to 37°C and 5% CO2. Researchers should wear PPE such as a lab coat, safety glasses, and gloves.
- Pre-warm a bottle of Spheroid Medium (SM) in a 37°C waterbath for at least 15 minutes prior to thawing the cells.
- Transfer 5mL of pre-warmed SM into a sterile conical (15-50ml), leaving the conical uncapped.
- Remove the cryovial and quickly submerge the vial in the 37°C waterbath.
Note: Do not submerge the cap, only the cell contents portion of the vial. - Allow the vial to thaw for 1.5 – 2 minutes or until a small spindle of ice is present in the cell suspension. DO NOT fully thaw the cell suspension.
- Remove the vial from the water bath and wipe thoroughly with an alcohol wipe.
- Remove the cap and transfer the cell suspension into the conical. Rinse the cryovial 2-3 times with warm SM from the conical to capture any remaining cells.
- Perform a trypan blue count using a 1:5 dilution (50µL of trypan blue + 350µL of SM + 100µL of cell suspension).
- Seed the wells with 1,000 – 5,000 cells per well using 100µL per well.
Note: These volumes are for using 96 well plate format. - Centrifuge the plate at 100xg for 2 minutes.
- Gently transfer the plate to the incubator (37°C, 5% CO2)
- Allow 4-7 days for the spheroids to form. Once the spheroids have formed (typically around days 4-6), perform a half-media change by carefully removing 50µL and then add 50µL of fresh warm SM per well.
- Perform half-media changes every 2-3 days thereafter.
3D Hepatocyte Spheroid Culture Results
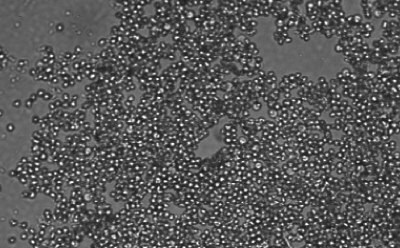
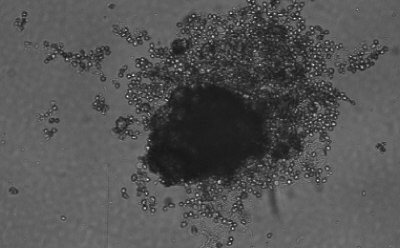
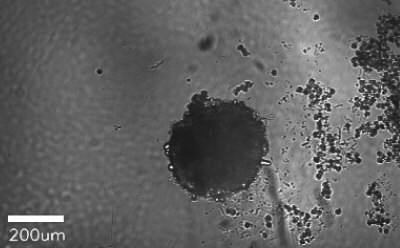
Figure 1. Spheroid-qualified Hepatocytes cultured for various days on ULA surface. Cells were seeded at 4,000 cells per well into 96 well ULA plate. Images were taken, a) right after seeding and centrifugation, b) after 3 days of culturing, and c) after 8 days of culturing. All images were taken using Millicell® Digital Cell Imager at 10x magnification.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?