All Photos(1)
About This Item
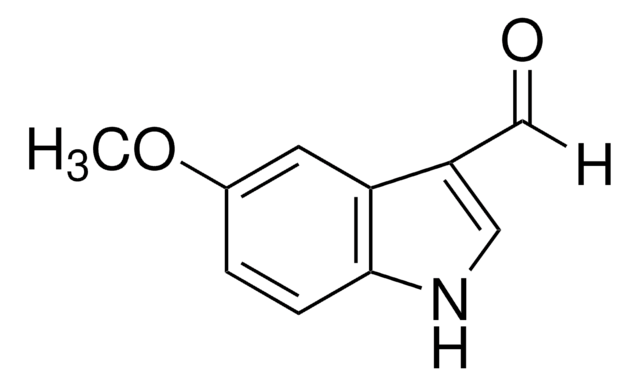
Empirical Formula (Hill Notation):
C9H6BrNO
CAS Number:
Molecular Weight:
224.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
204-207 °C (lit.)
SMILES string
[H]C(=O)c1c[nH]c2ccc(Br)cc12
InChI
1S/C9H6BrNO/c10-7-1-2-9-8(3-7)6(5-12)4-11-9/h1-5,11H
InChI key
PEENKJZANBYXNB-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S J Wratten et al.
Antimicrobial agents and chemotherapy, 11(3), 411-414 (1977-03-01)
An antibiotic-producing pseudomonad was isolated from a seawater sample from a La Jolla, Calif., tidepool. The pseudomonad produces two novel antibacterial compounds, 2-n-pentyl-4-quinolinol and 2-n-heptyl-4-quinolinol. It also synthesizes indole-3-carboxaldehyde, 6-bromoindole-3-carboxaldehyde, and the known antibiotic p-hydroxybenzaldehyde. Each of these compounds was
Elizabeth Almeida Lafayette et al.
European journal of medicinal chemistry, 136, 511-522 (2017-05-23)
Molecules bearing indole nucleus present diverse biological properties such as antitumor and anti-inflammatory activities that can be associated both to DNA and protein interactions. This study focused on the synthesis of new indole derivatives with thiazolidines and imidazolidine rings condensed
Sung Dae Cho et al.
Molecular cancer therapeutics, 7(7), 2109-2120 (2008-07-23)
Bis(3'-indolyl)methane (DIM) is a metabolite of the phytochemical indole-3-carbinol, and both compounds exhibit a broad spectrum of anticancer activities. We have developed a series of synthetic symmetrical ring-substituted DIM analogues, including 5,5'-dibromoDIM, which are more potent than DIM as inhibitors
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service