543896
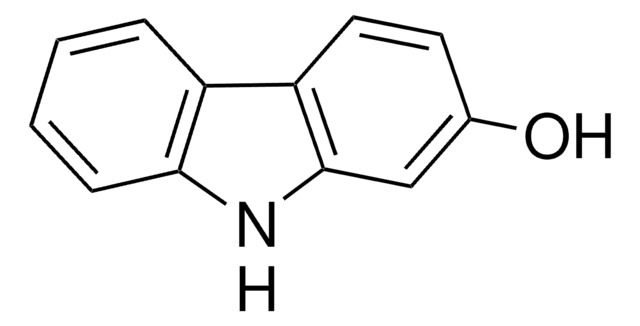
4-Hydroxycarbazole
95%
Synonym(s):
9H-Carbazol-4-ol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H9NO
CAS Number:
Molecular Weight:
183.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
169-173 °C (lit.)
SMILES string
Oc1cccc2[nH]c3ccccc3c12
InChI
1S/C12H9NO/c14-11-7-3-6-10-12(11)8-4-1-2-5-9(8)13-10/h1-7,13-14H
InChI key
UEOHATPGKDSULR-UHFFFAOYSA-N
Related Categories
General description
4-Hydroxycarbazole can be obtained from 1,2,3,4-tetrahydro-4-oxocarbazole via dehydrogenation with freshly prepared Raney nickel.
Application
4-Hydroxycarbazole may be used in the synthesis of the following:
It participates as an electron donor for the preparation of nonlinear optical (NLO) chromophores.
- 4-(2-bromoethoxy)-9H-carbazole
- 4-(3-bromopropoxy)-9H-carbazole
- 4-(4-bromobutoxy)-9H-carbazole
- 4-(5-bromopentyloxy)-9H-carbazole
- 4-(6-bromohexyloxy)-9H-carbazole
It participates as an electron donor for the preparation of nonlinear optical (NLO) chromophores.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kristan H Cleveland et al.
PloS one, 14(5), e0217038-e0217038 (2019-05-21)
Carvedilol is reported to prevent cancers in humans and animal models. However, a molecular mechanism has yet to be established, and the extent to which other β-blockers are chemopreventive remains relatively unknown. A comparative pharmacological approach was utilized with the
Pramod V Chavan et al.
Bioorganic chemistry, 85, 475-486 (2019-02-19)
A series of spirochromenocarbazole tethered 1,2,3-triazoles were synthesized via click chemistry based one-pot, five component reaction between N-propargyl isatins, malononitrile, 4-hydroxycarbazole, aralkyl halides and sodium azide using cellulose supported CuI nanoparticles (Cell-CuI NPs) as the heterogeneous catalyst. Antiproliferative activity of
Kevin M Huang et al.
Cancer prevention research (Philadelphia, Pa.), 10(10), 598-606 (2017-09-16)
In previous studies, the β-blocker carvedilol inhibited EGF-induced epidermal cell transformation and chemical carcinogen-induced mouse skin hyperplasia. As exposure to ultraviolet (UV) radiation leads to skin cancer, the present study examined whether carvedilol can prevent UV-induced carcinogenesis. Carvedilol absorbs UV
Synthesis, biological evaluation, and molecular modeling of berberine derivatives as potent acetylcholinesterase inhibitors.
Huang L, et al.
Bioorganic & Medicinal Chemistry, 18(3), 1244-1251 (2010)
Michela Rosini et al.
Journal of medicinal chemistry, 51(15), 4381-4384 (2008-07-09)
Alzheimer's disease (AD) is a multifactorial syndrome with several target proteins contributing to its etiology. To confront AD, an innovative strategy is to design single chemical entities able to simultaneously modulate more than one target. Here, we present compounds that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service