132292
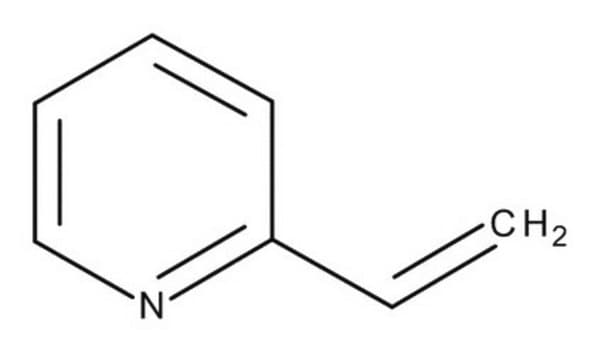
2-Vinylpyridine
97%
Synonym(s):
α-Vinylpyridine, 2-Ethenylpyridine, 2-Pyridylethylene
About This Item
Recommended Products
vapor pressure
10 mmHg ( 44.5 °C)
Assay
97%
form
liquid
contains
0.1 wt. % p-tert-butylcatechol as inhibitor
refractive index
n20/D 1.549 (lit.)
bp
79-82 °C/29 mmHg (lit.)
density
0.975 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
C=Cc1ccccn1
InChI
1S/C7H7N/c1-2-7-5-3-4-6-8-7/h2-6H,1H2
InChI key
KGIGUEBEKRSTEW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
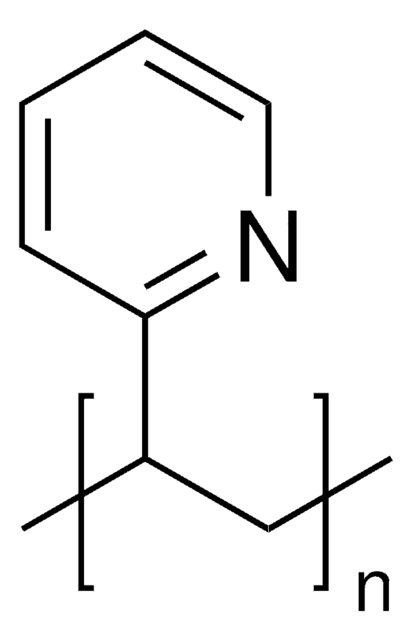
2-Vinylpyridine (2VP) is a water soluble pyridine derivative that can be used in the synthesis of poly(2-vinylpyridine) (P2VP) by anionic polymerization. It can be industrially prepared by treatment of 2-methylpyridine with an aqueous solution of formaldehyde in a temperature range of 150-200°C.
Application
- In the preparation of P2VP oligomers to study their properties and behavior on various surfaces, with a focus on area-selective deposition techniques.
- To synthesize pH-responsive thin film membranes, which can be used for various applications, such as separation, filtration, and catalysis.
- To synthesize degradable block copolymer-derived nanoporous membranes, which have potential applications in various fields, including energy-related catalysis and nanoparticle synthesis.
- To improve the effectiveness of protective polymer coatings on metals.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - Skin Sens. 1
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
122.0 °F - closed cup
Flash Point(C)
50 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service