89600
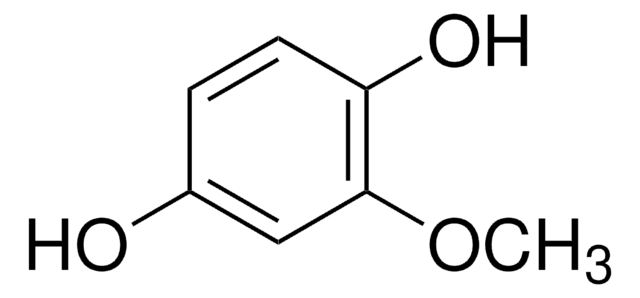
Methylhydroquinone
purum, ≥98.0% (HPLC)
Synonym(s):
Toluhydroquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H3-1,4-(OH)2
CAS Number:
Molecular Weight:
124.14
Beilstein:
2041489
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (HPLC)
form
crystals
autoignition temp.
851 °F
mp
126-128 °C
128-130 °C (lit.)
SMILES string
Cc1cc(O)ccc1O
InChI
1S/C7H8O2/c1-5-4-6(8)2-3-7(5)9/h2-4,8-9H,1H3
InChI key
CNHDIAIOKMXOLK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1A - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
341.6 °F - closed cup
Flash Point(C)
172 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Furihata et al.
Japanese journal of cancer research : Gann, 84(3), 223-229 (1993-03-01)
The possible tumor-promoting and tumor-initiating activities of p-methylcatechol and methylhydroquinone in the pyloric mucosa of male F344 rats were studied. Ornithine decarboxylase (ODC) and replicative DNA synthesis (RDS) were used as markers of tumor promotion and DNA single strand scission
Melissa García-Caballero et al.
Biochemical pharmacology, 85(12), 1727-1740 (2013-04-23)
Toluquinol, a methylhydroquinone produced by a marine fungus, was selected in the course of a blind screening for new potential inhibitors of angiogenesis. In the present study we provide the first evidence that toluquinol is a new anti-angiogenic-compound. In a
J W Priest et al.
Biochemistry, 28(23), 9192-9200 (1989-11-14)
A crude extract that catalyzes the epoxidation of toluquinol and gentisyl alcohol was isolated from cultures of Penicillium patulum. About 60% of the activity sedimented from crude extract upon centrifugation at 105,000g for 2 h, and at 30,000g for 30
Gönül Vardar et al.
Applied and environmental microbiology, 70(6), 3253-3262 (2004-06-09)
Toluene-o-xylene monooxygenase (ToMO) from Pseudomonas stutzeri OX1 oxidizes toluene to 3- and 4-methylcatechol and oxidizes benzene to form phenol; in this study ToMO was found to also form catechol and 1,2,3-trihydroxybenzene (1,2,3-THB) from phenol. To synthesize novel dihydroxy and trihydroxy
Montira Leelakriangsak et al.
Molecular microbiology, 67(5), 1108-1124 (2008-01-23)
Recently, we showed that the MarR-type repressor YkvE (MhqR) regulates multiple dioxygenases/glyoxalases, oxidoreductases and the azoreductase encoding yvaB (azoR2) gene in response to thiol-specific stress conditions, such as diamide, catechol and 2-methylhydroquinone (MHQ). Here we report on the regulation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service