33681
BDE No 100 solution
50 μg/mL in isooctane, analytical standard
Synonym(s):
2,2′,4,4′,6-Pentabromodiphenyl ether solution, 2,2′,4,4′,6-PentaBDE, PBDE 100
About This Item
Recommended Products
grade
analytical standard
Quality Level
shelf life
limited shelf life, expiry date on the label
concentration
50 μg/mL in isooctane
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
environmental
format
single component solution
storage temp.
2-8°C
SMILES string
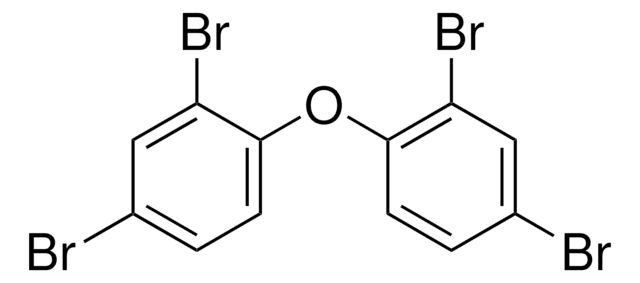
Brc1ccc(Oc2c(Br)cc(Br)cc2Br)c(Br)c1
InChI
1S/C12H5Br5O/c13-6-1-2-11(8(15)3-6)18-12-9(16)4-7(14)5-10(12)17/h1-5H
InChI key
NSKIRYMHNFTRLR-UHFFFAOYSA-N
General description
Application
- House dust samples using isotope dilution method combined with liquid chromatography coupled to negative ionization atmospheric pressure photoionization tandem mass spectrometry (LC-NI-APPI-MS/MS).
- Adipose tissue samples using gas chromatography coupled to ion-trap mass spectrometry (GC-IT-MS/MS).
Other Notes
The standard should be transferred to a clean and appropriate vial or flask using clean pipettes or micro pipettes. The vial should be immediately capped to avoid any loss or evaporation of the solvent.
After opening the ampoule, the standard should not be stored or kept in the ampoule. To preserve the integrity of the product, the standard should be transferred to an appropriate vial that must be capped and stored according to the recommendation on the Certificate of Analysis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
10.4 °F
Flash Point(C)
-12 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service