DABAL-Me3 - Non-pyrophoric Methyl Anion Equivalent
Introduction
Trimethylaluminum is a valuable reagent in organic synthesis due to its role both as a Lewis acid and a methyl anion equivalent. However, because of its pyrophoric nature, it cannot be easily handled in most laboratory settings. Developed by the Woodward group (University of Nottingham, U.K.), DABAL-Me3 is an adduct of trimethylaluminum and DABCO® that is a free-flowing solid that can be manipulated without the need for an inert atmosphere.1 The reagent has a hydrolytic stability comparable to LiBH4, as it is moisture sensitive but can be weighed out easily on the bench and stored in standard glassware. The reagent has been employed in numerous reactions including methylations of aldehydes and imines,1,2 the methylation of aryl and vinyl halides,3 conjugate additions to enones,4 and amide-bond formation.5 In the presence of the appropriate chiral catalyst, some of these transformations can be performed enantioselectively.
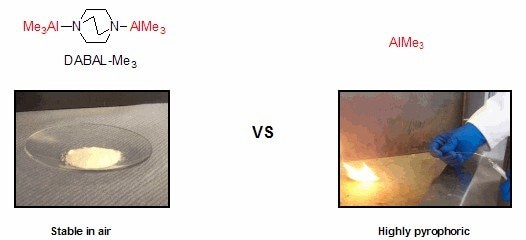

Figure 1. DABAL-Me3
Advantages
- Non-pyrophoric
- Excellent handling characteristics; solid can be handled briefly in non-humid air
- Useful methyl anion equivalent in numerous reaction paradigms
Representative Applications
Nickel-Catalyzed Addition to Aldehydes
DABAL-Me3 serves as a methyl anion source in nickel-catalyzed asymmetric additions of organoaluminum reagents to aldehydes. Electron-rich benzaldehyde derivatives give the highest enantioselectivities in this reaction. In certain cases, this method provides access to non-racemic chiral secondary alcohols not accessible through Noyori-type asymmetric transfer hydrogenation (e.g. 1-cyclohexylethanol).2,6
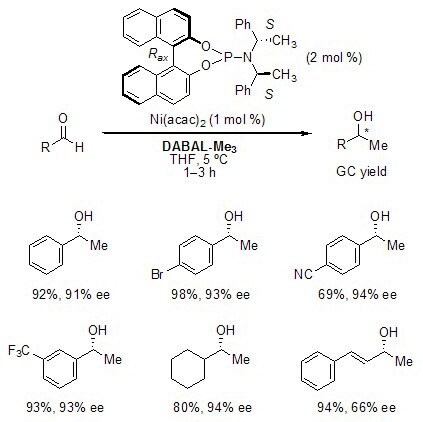
Figure 2. Nickel-Catalyzed Addition to Aldehydes
Methylation of Aryl and Vinyl Halides/Pseudohalides
DABAL-Me3 is a powerful methyl anion source in the Pd-catalyzed methylation of aryl and vinyl halides. The reactions generally provide the methylated product in >95% yield, and sensitive functional groups such as CN, OH, NO2, CO2R, and CHO are tolerant of DABAL-Me3.3
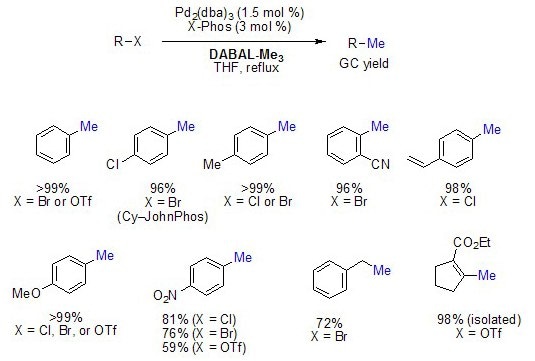
Figure 3. Methylation of Aryl and Vinyl Halides/Pseudohalides
Amide Bond Formation
Direct amide formation from unactivated esters and amines is typically achieved through generation of aluminum amides by treatment of the amine with trimethylaluminum or DIBAL. Reaction of the resultant metal amide with an ester delivers the amide. DABAL-Me3 serves as an excellent surrogate for these pyrophoric or difficult-to-handle aluminum reagents. A variety of functionalized amines and esters can be used in the amide bond forming reaction, including those bearing sensitive functional groups and stereocenters. Moreover, the reactions could be performed in open air using undried THF, with only a negligible decrease in reagent efficacy.5
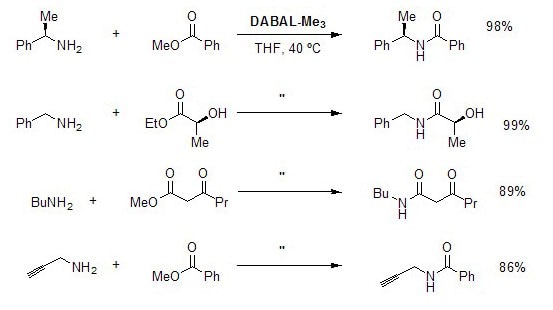
Figure 4. Amide Bond Formation
Other Reactions
DABAL-Me3 has also been used in copper(I)-catalyzed asymmetric additions of organoaluminum reagents to enones,1 as well as in asymmetric methylations of allylic electrophiles.2
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?