If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf
131377
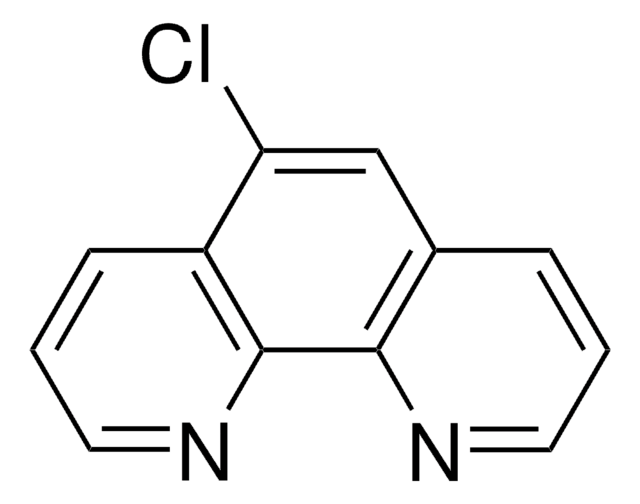
1,10-Phenanthroline
≥99%
Synonyme(s) :
o-phenanthroline
Sélectionner une taille de conditionnement
Sélectionner une taille de conditionnement
About This Item
Produits recommandés
Niveau de qualité
Essai
≥99%
Forme
powder
Pf
114-117 °C (lit.)
Chaîne SMILES
c1cnc2c(c1)ccc3cccnc23
InChI
1S/C12H8N2/c1-3-9-5-6-10-4-2-8-14-12(10)11(9)13-7-1/h1-8H
Clé InChI
DGEZNRSVGBDHLK-UHFFFAOYSA-N
Informations sur le gène
human ... FNTA(2339)
Catégories apparentées
Application
- A cathode buffer layer to improve the efficiency of organic solar cells.[1]
- A conventional chelator to study its efficacy in Fenton′s reaction-luminol chemiluminescence system.[2]
- A ligand in mild, copper (II)-catalyzed cross-coupling of organoboronic acids and sulfinate salts, leading to aryl- and alkenylsulfones.[3]
- A versatile ligand employed in the spectrophotometric determination of metals[4] and photocatalytic reduction of carbon dioxide.[5]
- A building block for metallomacrocycles.[6]
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
-
How can I determine the shelf life / expiration / retest date of this product?
1 réponse-
Utile ?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 réponse-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Utile ?
-
-
Do you have GMP pharmaceutical grade 1,10-Phenanthroline?
1 réponse-
Unfortunately, there are no GMP or pharmaceutical grade products available at this time.
Utile ?
-
-
What is the difference between 1,10-Phenanthroline Products 131377 and 320056?
1 réponse-
Product 320056 is the monohydrate form and 131377 has no specification on water content. Both can make a copper complex, since they are the same ligand.
Utile ?
-
-
What is the solubility of Product 131377, 1,10-Phenanthroline?
1 réponse-
1,10-Phenanthroline is soluble in methanol at 500 micrograms per mL, or in water at 3 milligrams per mL. It is also soluble in benzene and acetone.
Utile ?
-
-
Can Product 131377, 1,10-Phenanthroline, be used for cell culture?
1 réponse-
Sigma-Aldrich does not have any references for the use of 1,10-phenanthroline in cell culture.
Utile ?
-
-
What is the Department of Transportation shipping information for this product?
1 réponse-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Utile ?
-
Filtres actifs
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique