Ultrapure Water for BPA-free Culture Media
Merina Corpinot, PhD1, Maurizio Zuccotti, PhD2, Daniel Darbouret, PhD1
1R&D Department, Lab Water Solutions, Merck, Guyancourt, France, 2Laboratory of Developmental Biology, Department of Biology and Biotechnology “Lazzaro Spallanzani”, University of Pavia, Italy
This study’s objective was to establish a technique to detect low levels of the endocrine disruptor bisphenol A (BPA) in water and, more specifically, to document how a Milli-Q® IQ 7000 water purification system equipped with a Biopak® ultrafilter (configuration recommended for molecular biology applications) produces reference ultrapure water that is suitable for BPA-sensitive embryology research.
Read more about
- Health concerns of endocrine disruptors such as bisphenol A
- Study 1: HP-SPME-GC-MS to measure BPA in ultrapure water used in embryology research
- Study 2: BPA-free ultrapure water for cytological and molecular analyses to evaluate the impact of BPA on oocyte maturation
- Study 3: BPA-free ultrapure water for media used in cell-imaging technology
- Conclusion: Ultrapure water for BPA-sensitive applications in embryogenesis studies
- Materials and Methods for the HP-SPME-GC-MS analysis of BPA
- Related Products
Health concerns of endocrine disruptors such as bisphenol A
Endocrine disruptors are chemicals that interfere with the endocrine system of humans and animals. They affect growth as well as reproduction, immune and nervous systems, causing adverse health consequences. Found in plastics (including laboratory consumables and equipment), lacquers, resins and surfactants, endocrine disruptors are regarded as omnipresent.
Norms and standards regulate the maximum doses of endocrine disruptors allowed in food products and beverages as well as their presence in the environment. Among the various industrially produced endocrine disruptors, BPA is one of the most widespread. BPA is known as a "xenoestrogen" as it mimics the estrogen hormone, and therefore raises major health concerns. Several studies have confirmed that BPA interferes with the production of hormones and the expression of their genes. Recent research revealed that even low levels of BPA (20 µg/kg/day, which represents an environmentally-relevant BPA dose during 1-week of fetal exposure) can affect the production of healthy oocytes (Figure 1), by interfering during their growth inside the ovary.1-3
The goal of the studies described below was to demonstrate that the quality of ultrapure (Type 1) water obtained from a Milli-Q® purification system is suitable for preparing media and solutions that warrant the absence of BPA, specifically, a BPA-free culture environment for reproductive biology experiments.
- Study 1 shows the results of BPA analysis in different types of water using High-Performance Solid-Phase MicroExtraction coupled to Gas Chromatography-Mass Spectrometry (HP-SPME-GC-MS).
- Study 2 presents the results of cytological and molecular analyses to evaluate the impact of BPA on oocyte maturation.
- Study 3 shows the importance of water for culture media used in cell-imaging technology.
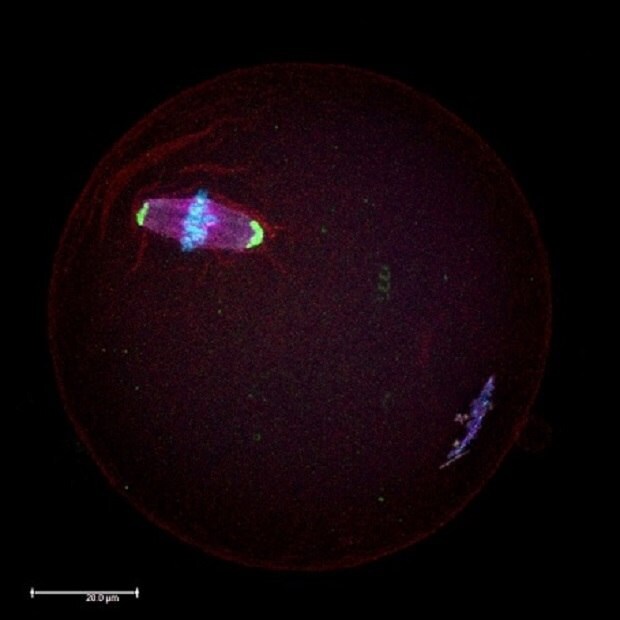
Figure 1.Microscopic immunocytochemistry analysis of a mouse oocyte during metaphase II (MII) meiotic stage using antibodies against proteins in the meiotic spindles (red) and centrosomes (green). Chromosomes were counterstained with DAPI (blue).
Study 1: HP-SPME-GC-MS to measure BPA in ultrapure water used in embryology research
In reproductive biology, water is a key reagent used to perform analyses (e.g., culture media preparation).
Scientists from the University of Pavia in Italy used a reproducible HP-SPME-GC-MS method to analyze the BPA content in the ultrapure water used in their embryological research. The analyzed ultrapure water was delivered from a Milli-Q® IQ 7000 ultrapure water purification system fed by Elix® pure water produced by a system that employs a combination of purification technologies, including advanced reverse osmosis (RO), Elix® electrodeionization (EDI) and a bactericidal UV lamp, similar to a Milli-Q® HX water system. The Milli-Q® IQ 7000 ultrapure system was equipped with a Biopak® polisher at the point of use (Figure 2). The Biopak® ultrafiltration polisher is recommended for molecular biology applications to deliver water that is free of endotoxins, nucleases, proteases, and bacteria.

Figure 2.Outline of the experimental analysis of Bisphenol A (BPA) in different laboratory water types using HP-SPME-GC-MS.
HP-SPME-GC-MS analysis showed that BPA was not detectable in the Elix® pure water nor the Milli-Q® ultrapure water used to feed the lab’s experiments, within a limit of detection (LOD) of 4 nM (Table 1).
As mentioned, experimental water was dispensed from a Biopak® polisher. Figure 3 shows a typical result obtained by analyzing three water samples dispensed consecutively from a Milli-Q® IQ 7000 system equipped with a Biopak® polisher at the point of use. The repeatability and the robustness of the GC-MS analysis, as well as the daily variations in BPA, were evaluated according to the procedures described in the materials and methods section below.
The results demonstrate that the ultrapure water quality obtained from the Milli-Q® IQ 7000 system does not contain detectable BPA (LOD = 4 nM). In particular, the analysis showed that BPA does not leach from the system into the product water, as it was not detectable in the tap water feeding the water purification system.
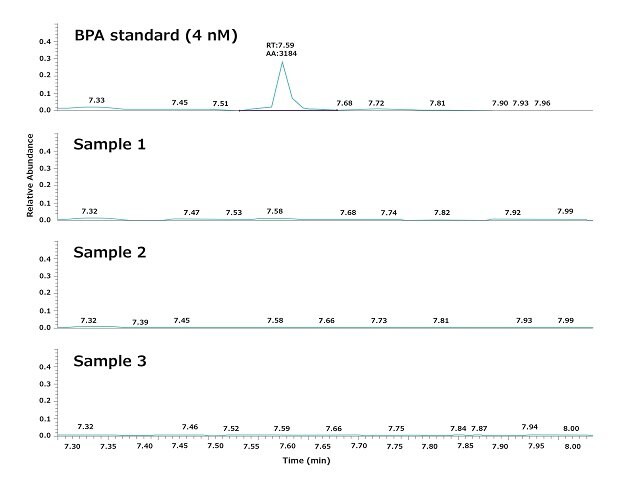
Figure 3.Chromatograms showing the levels of BPA in three ultrapure water samples. Samples were consecutively withdrawn at the point-of-use of a Milli-Q® IQ 7000 ultrapure water system equipped with a Biopak® polisher.
The following two studies reveal that a Milli-Q® ultrapure water purification system can be confidently used to dispense high-quality water with no BPA contamination and can be further employed in the study of the effects of endocrine disruptors on developing gametes and embryos, especially water withdrawn after the Biopak® polisher.
Study 2: BPA-free ultrapure water for cytological and molecular analyses to evaluate the impact of BPA on oocyte maturation
When measuring the impact of a particular molecule, the experimental environment must be free of the molecule of interest. In the presented study, scientists used qRT-PCR to analyze transcript expression of four maternal-effect genes, Brg1 (chromatin remodeler), Dnmt3a and Dnmt3l (DNA methylases), and Oct-4 (marker of cell pluripotency). Samples consisted of three independent pools of 10 metaphase II (MII) mouse oocytes, obtained following 15 hours in-vitro maturation (IVM) of fully-grown antral oocytes in the presence of 10 nM, 100 nM or 1000 nM BPA.
Results revealed that BPA did not arrest meiotic maturation at the three doses tested as the great majority (96.5%) of oocytes reached the MII stage, without a significant difference between controls (CTR) and exposed samples. However, the expression of all the genes tested was significantly altered (either higher or lower compared to untreated control) at all the BPA concentrations tested, except for Oct-4 that was unaltered at 10 nM BPA (Figure 4).
These gene expression experiments were performed using ultrapure water dispensed from a Milli-Q® IQ 7000 water purification system equipped with a Biopak® polisher. The results support the suitability of ultrapure water dispensed from this Milli-Q® system for use in BPA-sensitive applications in reproductive biology.
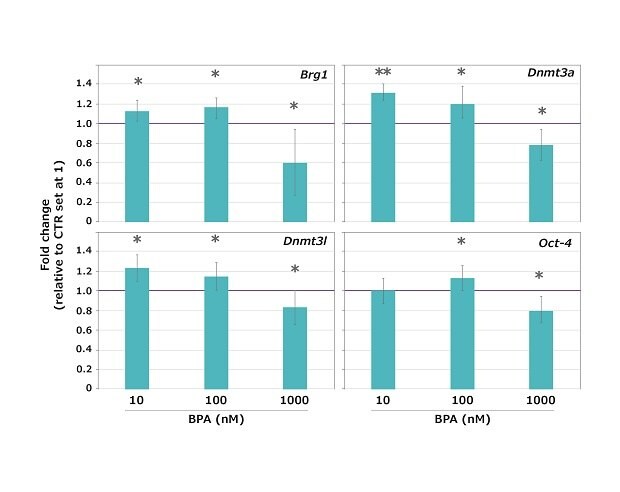
Figure 4.Expression profiles of the Brg1, Dnmt3a, Dnmt3l and Oct-4 maternal-effect genes in mouse oocytes exposed to 10, 100 or 1,000 nM BPA during the GV-to-MII transition. The expression values of control (CTR) samples were set at 1 for the calculation of the n-fold change. Values are expressed as mean ± SD. *p < 0.05 **p < 0.001.
Study 3: BPA-free ultrapure water for media used in cell-imaging technology
Time-lapse imaging allows us to observe the movements occurring inside an oocyte during its transition from the germinal vesicle (GV) to the MII stage of development (video4). These movements, known as Cytoplasmic Movement Velocity (CMV), serve as a non-invasive cytological marker to evaluate a female gamete developmental competence (a gamete’s quality and capacity to sustain further development).5,6 In the reported study, this novel technique has been used to investigate the CMV occurring during the GV-to-MII transition of oocytes matured in the absence or presence of increasing BPA concentrations (Figure 5 shows representative data for 100 nM BPA only).
Cell Particle Image Velocity (Cell PIV) analysis showed specific developmental time-windows when oocytes were sensitive to the BPA doses tested. Remarkably, when exposed to the low 10 nM BPA concentration, alterations in the CMV were dispersed at single time points, while at higher doses, the alterations in the CMV were visible during prolonged time-lapse during the GV-to-MI transition. Specifically, 100 nM BPA led to significant differences within a time interval when chromatin condenses, and centromeres aggregate into large chromocenters to form the MI plate (Figure 5).
The culture media involved in the above CMV experiments was prepared with ultrapure water delivered from a Milli-Q® IQ 7000 water purification system equipped with a Biopak® polisher. The results underline the need for BPA-free culture media as even low 100 nM doses of BPA may have effects, detected either through the analysis of gene expression or of cytoplasmic movements.
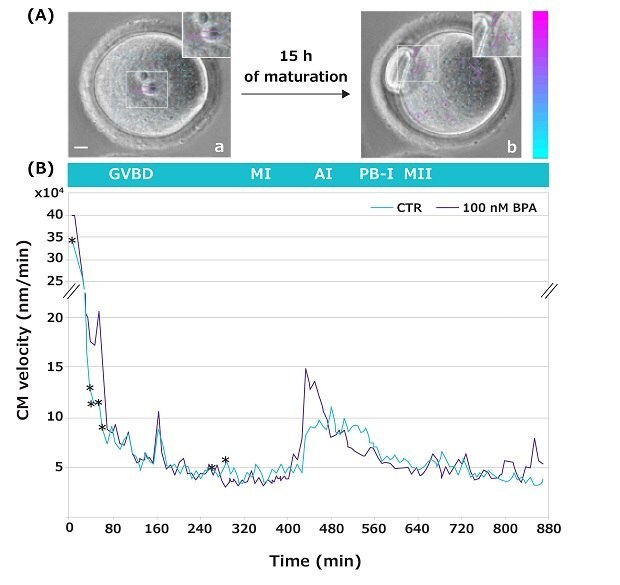
Figure 5.(A) Representative images of a GV oocyte, taken at frame 1 (a), and of an MII oocyte at frame 100 (b). Insets show magnified images with arrows (velocity vectors) indicating the intensity and direction of cytoplasmic movements. Color and length of the arrows indicate the velocity module of the movements compared to the previous frame. Colored vector scale bar (nm/min): blue indicates low velocity; purple indicates high velocity. Bar: 10 μm. (B) Cytoplasmic movement profile during GV-to-MII transition of mouse oocytes in the absence (green) or presence (gray) of 100 nM BPA. *p < 0.05.
Conclusion: Ultrapure water for BPA-sensitive applications in embryogenesis studies
The increasing sensitivity and performance of research techniques requires high-quality purified water to prevent contamination that can compromise the accuracy and authenticity of experimental results. Especially in studies involving high-breakthrough investigative tools, scientists require high-purity water to prevent any challenges that can disturb their results (e.g., BPA, endotoxins, nucleases, proteases and bacteria) and for greater confidence in their experimental analyses.
In the field of embryology, even low levels of the endocrine disruptor BPA have been shown to compromise embryogenesis research by interfering with oocytes growth. This is concerning because BPA, although not necessarily originally present in a lab’s tap feed water, can leach from laboratory consumables and equipment, causing interferences with experimental results.
In this article, although we showed that low BPA levels did not arrest meiotic maturation, a very low BPA concentration of only 10 nM impacted both gene expression and the movements occurring inside an oocyte during its transition from the germinal vesicle (GV) to the MII stage of development. These results highlight the need to perform embryology experiments in a BPA-free environment, including the use of ultrapure water that is assured to have non-detectable levels of BPA.
Ultrapure water delivered from a Milli-Q® IQ 7000 ultrapure water purification system equipped with Biopak® polisher was shown to not contain detectable BPA, at an even very low detection limit of only 4 nM according to the method used in this paper. The ultrapure water delivered by this system can thus be confidently used to study the effects of endocrine disruptors on developing gametes and embryos.
To find water purification solutions adapted to the needs of BPA-sensitive analyses, request support from a lab water expert.
Or, select and configure the optimal water purification system for your lab.
Materials and Methods for the HP-SPME-GC-MS analysis of BPA
Instruments
- A Thermo Scientific DSQII single quadrupole GC/MS system (TraceDSQII mass spectrometer, TraceGCUltra gas chromatograph) equipped with a CombiPAL autosampler (CTC Analytics, Switzerland) was used to perform water analysis.
- A 30 mm length, 0.25 I.D., 0.25 µm film thickness Restek RxiTM - 5ms capillary column (5% diphenyl/95%dimethyl polysilane – Restek Corporation, Bellefonte, USA) was used with helium as the carrier gas at a constant flow rate of 1.0 mL/min.
- A 100 µm polydimethylsiloxane (PDMS) SPME fiber assembly was used. The fiber was cleaned before each analysis for 20 min in the needle heater at 250 °C to avoid carryover from previous analysis.
Standard Preparation
A BPA stock solution at 10 nM concentration was prepared in 100% ethanol. A series of dilutions was made to obtain a calibration curve with four different concentration levels, 1, 2, 4, 40 nmol/L using HPLC water.
Experiment 1: Evaluation of Repeatability and Robustness of the GC-MS Analysis
Three water samples (5 mL) were consecutively collected from each of three sources: tap water, Elix® pure water, and at the point-of-use of a Milli-Q® IQ 7000 ultrapure water system equipped with a Biopak® polisher. Before collection, 5 L of water were dispensed to flush the system.
Experiment 2: Evaluation of Daily Variations in BPA Concentration
Three water samples (5 mL) were collected from tap water, Elix® pure water, and at the point-of-use of a Milli-Q® IQ 7000 ultrapure water system equipped with a Biopak® polisher, every 24 h for three days. Before collection, 5 L of water were dispensed. Chromatograms are available on-demand.
Water Samples
A volume of 5 mL of water to be analyzed (referred to as tap water, Elix® pure water and Biopak®-filtered ultrapure water) was added into 20 mL glass vials. In each vial, 200 mg KHCO3, 1 g NaCl and a disposable magnetic stirring bar were added.
Derivatization
30 µL of acetic anhydride was added to the water sample and vials were sealed with a headspace aluminum cap with a polytetrafluoroethylene-faced septum. A complete reaction was obtained with a reaction time of derivatization of 5 min at 80 °C (with continuous agitation at 50 rpm). The headspace extraction of BPA was performed with the 100 µm PDMS fiber at 80 °C for 30 min with agitation (500 rpm). The SPME fiber was desorbed in the injector at 250 °C for 2 min.
Chromatographic Analysis
The oven temperature was held at 150 °C for 2 min, increased by 30 °C/min to 280 °C and held for 6 min. The injection was performed in splitless mode, splitless time was 2 min and the PTV injector temperature was held at 250 °C. The transfer line was kept at 290 °C and the ion source at 250 °C. MS was operated in the electron impact ionization (EI) mode and the ions were registered both in full scan mode, with a mass range of m/z 35-350 amu, and in SIM (selected monitoring) mode, using the m/z 270 as quantifier ion for BPA.
Acknowledgements
The authors thank the laboratory of developmental biology of the University of Pavia (Italy) for carrying out the present research and our R&D colleagues for their contribution to this project.
References
To continue reading please sign in or create an account.
Don't Have An Account?