512125
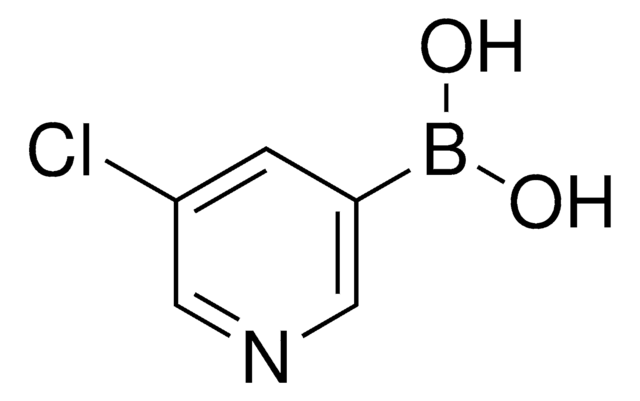
3-Pyridinylboronic acid
≥95.0%
Synonym(s):
3-Pyridineboronic acid, 3-Pyridylboronic acid, Dihydroxy(3-pyridyl)borane, Pyridin-3-ylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C5H6BNO2
CAS Number:
Molecular Weight:
122.92
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
mp
>300 °C (lit.)
SMILES string
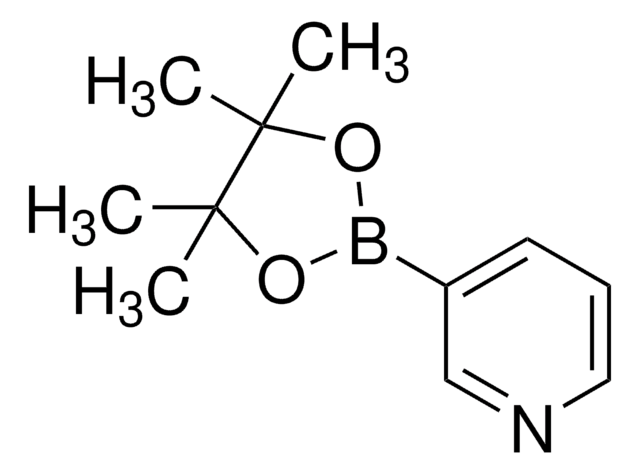
OB(O)c1cccnc1
InChI
1S/C5H6BNO2/c8-6(9)5-2-1-3-7-4-5/h1-4,8-9H
InChI key
ABMYEXAYWZJVOV-UHFFFAOYSA-N
Related Categories
Application
3-Pyridinylboronic acid can be used as a reagent for:
It can also be used to prepare:
- Phosphine-free Suzuki-Miyaura cross-coupling reactions.
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.
- N-arylation using copper acetylacetonate catalyst.
- Copper-mediated cyanation and regioselective cyanation of electron-rich benzenes.
It can also be used to prepare:
- New linear poly(phenylpyridyl) chains by Suzuki coupling.
- Oligopyridyl foldamers as mimics of a-helix twist.
- Many highly significant therapeutic enzymatic and kinase inhibitors and receptor antagonists.
- Pyridine substituted pyridinium N-(2′-azinyl)aminides by reacting with dibromo pyridinium aminides via Suzuki coupling reaction.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Brian C Shook et al.
Journal of medicinal chemistry, 55(3), 1402-1417 (2012-01-14)
The design and characterization of two, dual adenosine A(2A)/A(1) receptor antagonists in several animal models of Parkinson's disease is described. Compound 1 was previously reported as a potential treatment for Parkinson's disease. Further characterization of 1 revealed that it was
Synthesis of new linear poly(phenylpyridyl) chains
Perato, S.; et al.
Tetrahedron, 68, 1910-1917 (2012)
Justin I Montgomery et al.
Journal of medicinal chemistry, 55(4), 1662-1670 (2012-01-20)
The synthesis and biological activity of a new series of LpxC inhibitors represented by pyridone methylsulfone hydroxamate 2a is presented. Members of this series have improved solubility and free fraction when compared to compounds in the previously described biphenyl methylsulfone
Jana Sopkova-de Oliveira Santos et al.
Journal of chemical information and modeling, 52(2), 429-439 (2011-12-27)
Protein-protein interactions are central to many biological processes, from intracellular communication to cytoskeleton assembly, and therefore represent an important class of targets for new therapeutics. The most common secondary structure in natural proteins is an α-helix. Small molecules seem to
Cu(acac)2-catalyzed N-arylations of phenylurea with arylboronic acid
Gavade, S.; et al.
Synthetic Communications, 42, 1704-1714 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service