All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C26H41N3O5
CAS Number:
Molecular Weight:
475.62
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
Assay
≥98%
Quality Level
solubility
DMSO or DMF: 25 mg/mL
storage temp.
−20°C
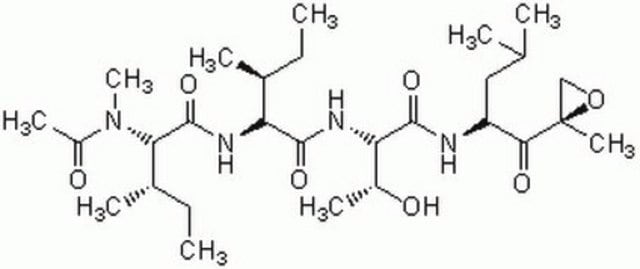
SMILES string
CC(C)C[C@@H](C(N[C@H](C(N[C@H](C=O)CC(C)C)=O)CC(C)C)=O)NC(OCC1=CC=CC=C1)=O
InChI
1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1
InChI key
TZYWCYJVHRLUCT-VABKMULXSA-N
Application
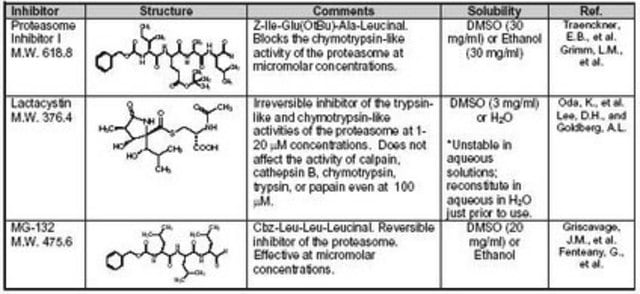
(R)-MG132 has been used in ubiquitination assay and is used as a proteasome inhibitor.
Biochem/physiol Actions
MG132 (carbobenzoxy-Leu-Leu-leucinal) is a tri-peptide aldehyde. It possesses antitumor activity and boosts cytostatic/cytotoxic effects of chemo- and radiotherapy. (R)-MG132 is a potent, membrane-permeable proteasome inhibitor. It can inhibit proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells. The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities than the (S)-MG132.
Physical form
crystalline solid or supercooled liquid
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Mad2-Mediated Translational Regulatory Mechanism Promoting S-Phase Cyclin Synthesis Controls Origin Firing and Survival to Replication Stress.
Gay S, et al.
Molecular Cell, 70(4), 628-638 (2018)
Alternative promotion and suppression of metastasis by JNK2 governed by its phosphorylation.
Hu S, et al.
Oncotarget, 8(34), 56569-56569 (2017)
Studies of the synthesis of all stereoisomers of MG-132 proteasome inhibitors in the tumor targeting approach.
Mroczkiewicz M, et al.
Journal of Medicinal Chemistry, 53(4), 1509-1518 (2010)
Activation of anaphase-promoting complex by p53 induces a state of dormancy in cancer cells against chemotherapeutic stress.
Dai Y, et al.
Oncotarget, 7(18), 25478-25478 (2016)
Yanling Tao et al.
Oncology letters, 14(6), 6365-6372 (2017-11-23)
Osteosarcoma (OS) is among the most frequently occurring bone tumors, particularly in children. Clinical treatment of OS is limited due to several factors including resistance to chemotherapy drugs and metastasis, and the underlying molecular mechanisms remain unclear. In the present
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service