All Photos(2)
About This Item
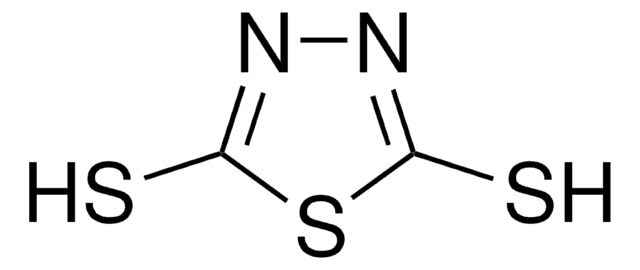
Empirical Formula (Hill Notation):
C11H11NO2
CAS Number:
Molecular Weight:
189.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
83-85 °C (lit.)
SMILES string
CC1(CC(=O)NC1=O)c2ccccc2
InChI
1S/C11H11NO2/c1-11(7-9(13)12-10(11)14)8-5-3-2-4-6-8/h2-6H,7H2,1H3,(H,12,13,14)
InChI key
UDESUGJZUFALAM-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Arcadius V Krivoshein
ACS chemical neuroscience, 7(3), 316-326 (2016-01-08)
Although the antiepileptic properties of α-substituted lactams, acetamides, and cyclic imides have been known for over 60 years, the mechanism by which they act remains unclear. I report here that these compounds bind to the nicotinic acetylcholine receptor (nAChR) and
J C Gomora et al.
Molecular pharmacology, 60(5), 1121-1132 (2001-10-20)
Inhibition of T-type Ca(2+) channels has been proposed to play a role in the therapeutic action of succinimide antiepileptic drugs. Despite the widespread acceptance of this hypothesis, recent studies using rat and cat neurons have failed to confirm inhibition of
M B Tennison et al.
Pediatrics, 87(2), 186-189 (1991-02-01)
Methsuximide was added to the therapeutic regimens of 25 children with intractable epilepsy. In 15 patients the drug was well tolerated and resulted in a 50% or greater reduction in seizure frequency. No serious or irreversible adverse effects were seen.
Pharmacokinetics of N-desmethylmethsuximide in pediatric patients.
M V Miles et al.
The Journal of pediatrics, 114(4 Pt 1), 647-650 (1989-04-01)
G K Szabo et al.
Clinical chemistry, 28(1), 100-104 (1982-01-01)
We describe an improved "high-pressure" liquid-chromatographic assay for simultaneous determination in serum of the five major antiepileptic drugs (ethosuximide, primidone, phenobarbital, phenytoin, and carbamazepine) and N-desmethylmethsuximide (the compound that must be quantitated for therapeutic drug monitoring of the antiepileptic drug
Chromatograms
application for HPLCapplication for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service