479179
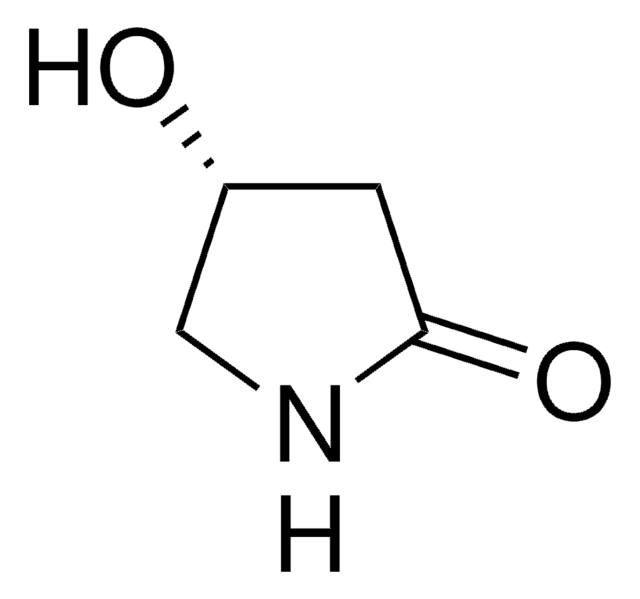
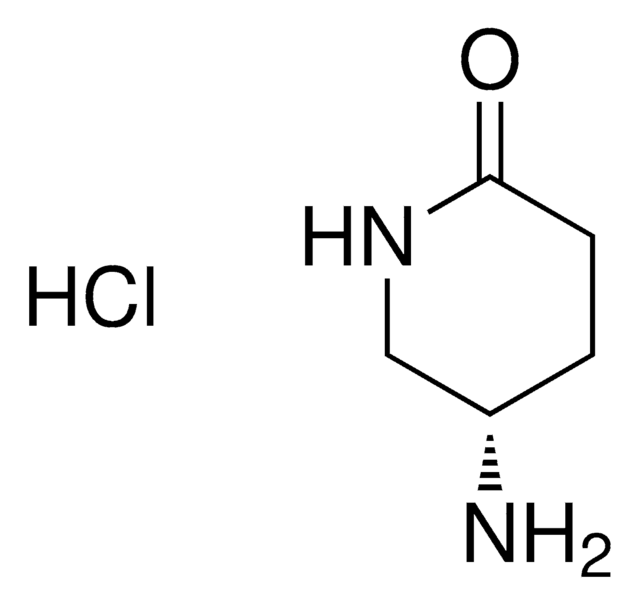
(S)-(−)-4-Hydroxy-2-pyrrolidinone
97%
Synonym(s):
(S)-β-Hydroxy-γ-butyrolactam
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7NO2
CAS Number:
Molecular Weight:
101.10
Beilstein:
1524192
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]23/D −43°, c = 1 in ethanol
mp
156-159 °C (lit.)
SMILES string
O[C@@H]1CNC(=O)C1
InChI
1S/C4H7NO2/c6-3-1-4(7)5-2-3/h3,6H,1-2H2,(H,5,7)/t3-/m0/s1
InChI key
IOGISYQVOGVIEU-VKHMYHEASA-N
Related Categories
General description
4-Hydroxy-2-pyrrolidinone is an important building block found in many bioactive compounds like streptopyrrolidine. It can be used as an intermediate in the synthesis of various γ-amino acids (GABA) and substituted 2-pyrrolidinones like cynometrine and cynodine.
Application
(S)-(−)-4-Hydroxy-2-pyrrolidinone can be used as a starting material in the preparation of:
- Biologically significant pyrrolo[1,2:1′,2′]azepino[5,6-b]indole derivatives.
- Substituted azepanes by reacting with various aldehydes via photochemical [5+2] cycloaddition.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Photochemical Two-Step Formal [5+ 2] Cycloaddition: A Condensation-Ring-Expansion Approach to Substituted Azepanes
Thullen SM, et al.
Synlett, 28(20), 2755-2758 (2017)
Streptopyrrolidine, an angiogenesis inhibitor from a marine-derived Streptomyces sp. KORDI-3973
Shin HJ, et al.
Phytochemistry, 69(12), 2363-2366 (2008)
First synthesis of pyrrolo [1, 2: 1′, 2′] azepino [5, 6-b] indole derivatives
Perron J, et al.
Tetrahedron Letters, 44(35), 6553-6556 (2003)
G Di Silvestro et al.
Journal of pharmaceutical sciences, 82(7), 758-760 (1993-07-01)
The phase diagram of (R)- and (S)-4-hydroxy-2-pyrrolidone presents a conglomerate in the racemic mixture. The enthalpy of melting extrapolated by the Schröder-van Laar-Le Chatelier equation [change in enthalpy (delta H) = 28410 J/mol; melting temperature (TA) = 429.9 K; solidus
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service