All Photos(1)
About This Item
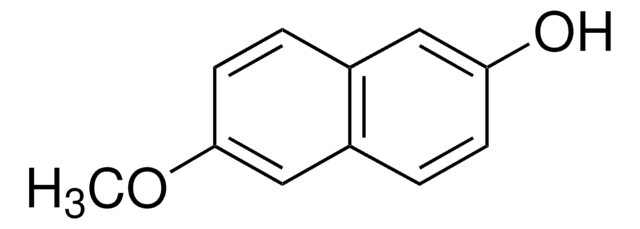
Linear Formula:
CH3OC10H6OH
CAS Number:
Molecular Weight:
174.20
Beilstein:
2207279
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
116-119 °C (lit.)
SMILES string
COc1ccc2ccc(O)cc2c1
InChI
1S/C11H10O2/c1-13-11-5-3-8-2-4-10(12)6-9(8)7-11/h2-7,12H,1H3
InChI key
UNFNRIIETORURP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
7-Methoxy-2-naphthol is a 7-substituted-2-naphthol. Crystal structure of 7-methoxy-2-naphthol has been reported. Reaction of 7-methoxy-2-naphthol with N-methyl-N-phenylhydrazine under thermal conditions has been reported.
Application
7-Methoxy-2-naphthol may be used in the synthesis of new 1H-benzo[f]chromene derivatives. It may be used in the synthesis of 7-methoxy-2-naphthyl (MN)-derived xylosides in which the methoxy group served as a marker for NMR characterization and UV detection.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 9-methoxy and 9-acetoxy-3-amino-1-(4-methoxyphenyl)-1H-benzo [f] chromene-2-carbonitriles via 2-(imino-piperidin-1-yl-methyl)-3-(4-methoxyphenyl) acrylonitrile as intermediate.
El-Agrody AM, et al.
Zeitschrift fur Naturforschung, B: Chemical Sciences, 57(5), 579-585 (2002)
Sandrine Gulberti et al.
The Journal of biological chemistry, 280(2), 1417-1425 (2004-11-04)
We determined whether the two major structural modifications, i.e. phosphorylation and sulfation of the glycosaminoglycan-protein linkage region (GlcAbeta1-3Galbeta1-3Galbeta1-4Xylbeta1), govern the specificity of the glycosyltransferases responsible for the biosynthesis of the tetrasaccharide primer. We analyzed the influence of C-2 phosphorylation of
7-Methoxy-2-naphthol.
Prince P, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 47(10), 2218-2220 (1991)
Qiang Tang et al.
Journal of the American Chemical Society, 130(18), 5840-5841 (2008-04-15)
An unexpected reaction of N,N-disubstituted hydrazine with naphthol and its analogues under simply thermal conditions has been disclosed. 2-Naphthol reacted with various N,N-disubstituted hydrazines under argon to afford 1-amino-2-naphthol and the corresponding secondary amines in excellent yields. Ortho amination of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service