Safinamide Impurity Profiling by HPLC Assay Methods
Neil J. Williams1, Jenna Milliken1
Merck
INTRODUCTION
A simple and accurate method was developed for purity analysis of safinamide using high-performance liquid chromatography with a diode array detector (HPLC-DAD). The method was developed for a family of safinamide compounds consisting of the active pharmaceutical ingredient safinamide and multiple corresponding impurities. This is to our knowledge the first reported method for safinamide and related impurities. Safinamide free acid, safinamide mesylate, safinamide defluor-derivative, and safinamide imidazolidinone can be resolved in a 20-minute method using an Ascentis® Express C8 column (150 x 4.6 mm, 5 μm). A gradient that utilized 0.1% trifluoroacetic acid in water and acetonitrile was employed. Under the applied conditions the system suitability criteria are met. The method demonstrates good resolution and selectivity, reproducibility, and sensitivity.
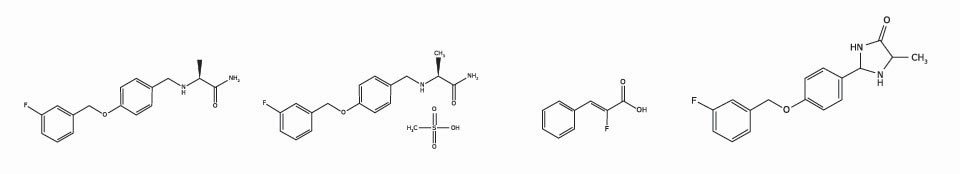
Safinamide Free Acid
PHR2380
Safinamide Mesylate
PHR2379
Safinamide Deflour-Derivative
PHR2381
Safinamide Imidazolidinone
PHR2382
Figure 1.Blank chromatogram.
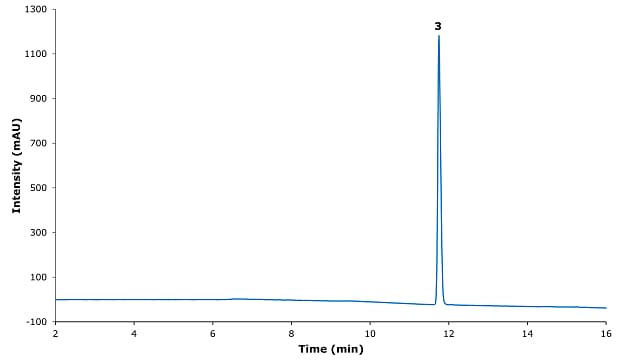
Figure 2.Chromatogram for safinamide mesylate system suitability solution.
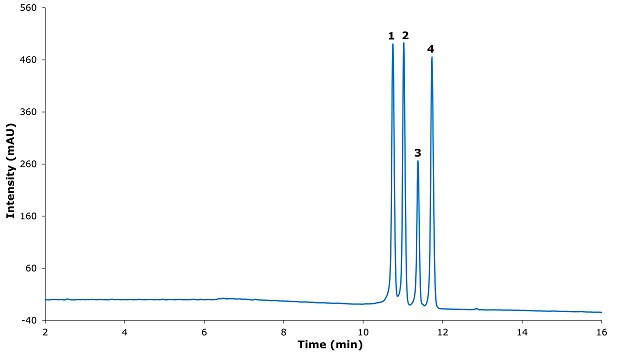
Figure 3.Chromatogram for resolution solution.
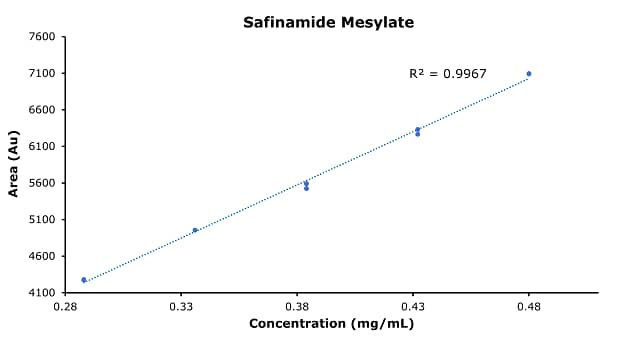
Figure 4.Calibration curve of safinamide mesylate.
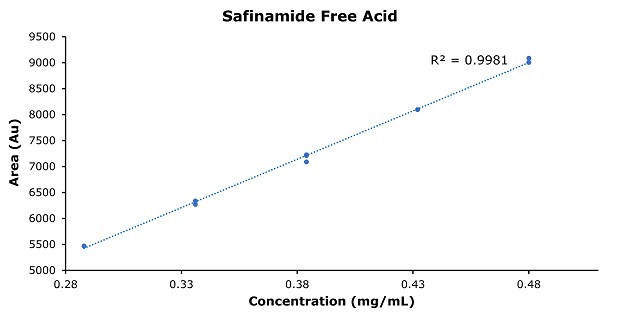
Figure 5.Calibration curve of safinamide free acid.

Figure 6.Calibration curve of safinamide defluro-derivative.
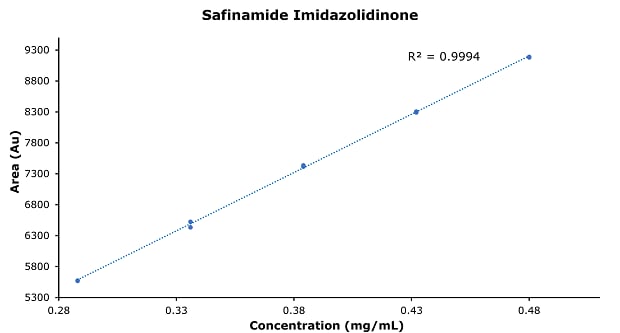
Figure 7.Calibration curve of safinamide imidazolidinone.
CONCLUSION
A new method was developed for analyzing the purity of safinamide and its related compounds using high-performance liquid chromatography with a diode array detector. The method uses an Ascentis® Express C8 HPLC column and a gradient of 0.1% trifluoroacetic acid in water and acetonitrile and takes 20 minutes to resolve the compounds. The method is reproducible and sensitive and shows good resolution and selectivity.
See also Small Molecules Analysis & Quality Control (QC) for more information on other applications or use our chromatogram search.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?