cataCXium® Catalysts
Introduction
Cross-coupling reactions are an important class of catalytic transformations with applications in polymer science as well as in the fine chemicals and pharmaceutical industries. The use of aryl chlorides for these transformations is preferred due to their low cost and their availability. However, despite the tremendous progress made in catalyst design, the Pd loading for these reactions
(1–3 mol%) prohibits industries to using them on a kilogram scale. The quest to synthesize a very active catalyst with very low loading has been ongoing for the past 20 years.
- Atom economical method
- High yields
- High turnover numbers
Suzuki Coupling with Low Loading of Catalyst
Beller and co-workers developed a very bulky ligand based on diadamantylalkylphosphanes.1,2,3 Among the most active ligand synthesized by this group, diadamantyl-n-butylphosphane, proved to be the most efficient. Using 0.005 mol% of Pd(OAc)2 and 0.01 mol% of phosphine, good to excellent yields were reported (58–100%) (Scheme 1). It is important to note that ortho substituents on the aryl chloride are tolerated and that only strongly electron donating substituents such as the methoxy group led to a drop in yields (60%) (Table 1).

Scheme 1
Evonik in collaboration with professor Beller and co-workers, developed a novel class of phosphine ligands, the phosphino-substituted N-aryl pyrroles.4 These biaryl ligands are sterically demanding and electron rich. When applied to the reaction of a variety of aryl chlorides with phenyl boronic acid, they showed very high turnover numbers at mild reaction temperatures. Low catalyst loading (0.05 mol%) was needed for the cross-coupling of a variety of aryl chlorides with phenylboronic acid (Scheme 2). Utilizing this ligand, with Pd(OAc)2 or Pd2(dba)3, for the conversion of electron rich and electron deficient aryl chlorides, resulted in excellent yields. Table 2 summarizes the yield reported for a variety of aryl chlorides reacted with phenylboronic acid using 0.01 mol% of Pd2(dba)3 at 60 °C.

Scheme 2
Building on the success synthesizing phosphino-N-aryl pyrroles, Beller and coworkers developed a similar ligand. They synthesized phosphino-N-phenylindole based ligands.5 To assess the reactivity of these ligands, they carried out the cross-coupling reaction of chlorobenzene with aniline (Scheme 3). This new system proved to be the best among the different ligands developed by Beller and co-workers for this specific cross-coupling reaction. Using Pd(OAc)2 and the tert-butyl phosphine phenyl indole ligand with NaOtBu as a base in toluene, a wide variety of amines were arylated with excellent yields.
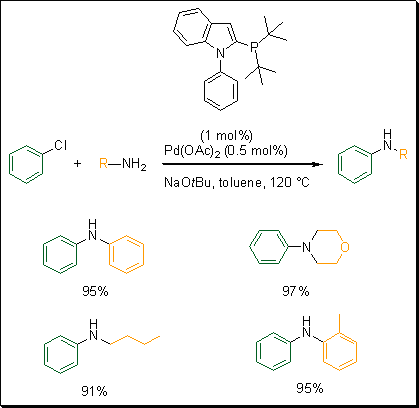
Scheme 3
References
Cross-coupling Reactions
A new class of phosphorus ligand was developed by Plenio and co-workers based on fluorenylphosphines architecture.1 To test the potency of these new ligands, the team of scientists carried out several common cross-coupling reactions: Suzuki, Sonogashira and Buchwald–Hartwig amination (Figure 1). The ligand proved to be very active for the Suzuki coupling of a variety of aryl chlorides with 4-methylphenylboronic acid with loading of 0.05 to 0.5 mol% of palladium. The electron donating substituent such as OMe did not affect the yields with >99% conversion. The Sonogashira transformation was carried out with a variety of aryl chloride coupled to phenylacetylene. With 1 mol% loading of catalyst, 44% to 94% yields were reported. Finally, the Buchwald–Hartwig amination results were very promising with quantitative conversion reported for several reactions with catalyst loading as low as 0.1 mol%.
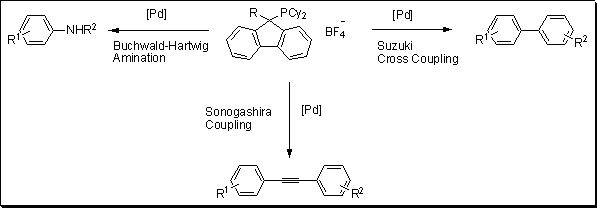
Figure 1.
References
Heck Catalysis
Hermann and co-workers developed a new class of palladium catalyst called palladacycles.1 These complexes are thermally stable up to 100 °C and are very potent for Heck catalysis with TON > 10,000. Tietze et al. utilized the activity of this catalyst to synthesize a derivative of cephalostatin, a steroid that showed potency against cancer cells.2 A double coupling reaction was carried out with an overall yield of 80%.

References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?