120847
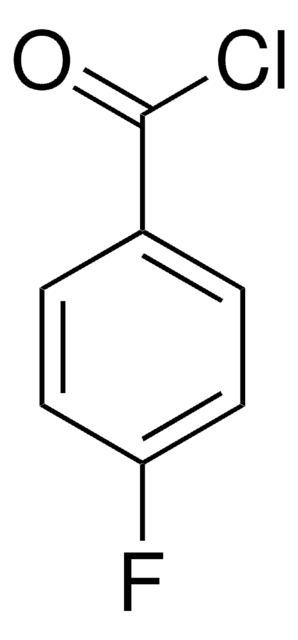
2-Fluorobenzoyl chloride
99%
About This Item
Productos recomendados
Análisis
99%
índice de refracción
n20/D 1.536 (lit.)
bp
90-92 °C/15 mmHg (lit.)
mp
4 °C (lit.)
densidad
1.328 g/mL at 25 °C (lit.)
cadena SMILES
Fc1ccccc1C(Cl)=O
InChI
1S/C7H4ClFO/c8-7(10)5-3-1-2-4-6(5)9/h1-4H
Clave InChI
RAAGZOYMEQDCTD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Skin Corr. 1B
Código de clase de almacenamiento
8A - Combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
251.6 °F - closed cup
Punto de inflamabilidad (°C)
122 °C - closed cup
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico