All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H10O2
CAS Number:
Molecular Weight:
138.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.485 (lit.)
bp
62 °C/0.25 mmHg (lit.)
density
1.038 g/mL at 25 °C (lit.)
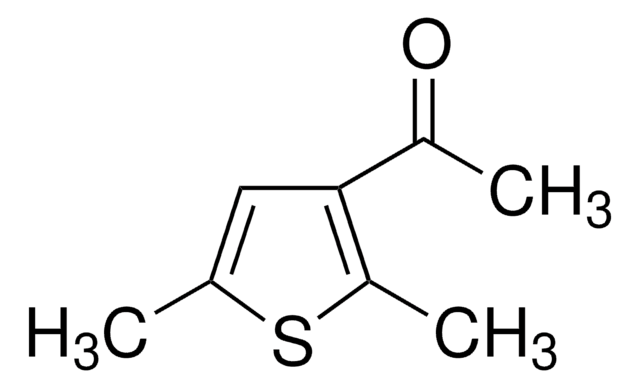
SMILES string
CC(=O)c1cc(C)oc1C
InChI
1S/C8H10O2/c1-5-4-8(6(2)9)7(3)10-5/h4H,1-3H3
InChI key
KBSVBCHYXYXDAG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Acetyl-2,5-dimethylfuran is a flavouring agent and its specifications were revised.
Application
3-Acetyl-2,5-dimethylfuran was used in synthesis of:
- furylfulgide, 2-[1-(2, 5-dimethyl-3-furyl) ethylidene]-3-isopropylidene succinic anhydride
- chalcones
- 1-(2′,5′-dimethyl-3′-furyl)-3-(aryl)-2-propen-1-one
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Bend et al.
World Health Organization technical report series, (947)(947), 1-225 (2008-06-17)
This report represents the conclusions of a Joint FAO/WHO Expert Committee convened to evaluate the safety of various food additives, including flavouring agents, with a view to recommending acceptable daily intakes (ADIs) and to preparing specifications for identity and purity.
Photochromism of a furylfulgide, 2-[1-(2, 5-dimethyl-3-furyl) ethylidene]-3-isopropylidene succinic anhydride in solvents and polymer films
Yokoyama Y, et al.
Bulletin of the Chemical Society of Japan, 63(6), 1607-1610 (1990)
SYNTHESIS AND STUDY OF ANTI-INFLAMMATORY AND ANTIMICROBIAL ACTIVITY OF SOME NEW 1, 3, 5-TRISUBSTITUTED PYRAZOLINES.
Sridhar S, et al.
International Journal of Chemical Sciences, 8(4), 2697-2707 (2010)
Maura Koehle et al.
ChemSusChem, 10(1), 91-98 (2016-12-13)
A four-step catalytic process was developed to produce p-methylstyrene from methylfuran, a biomass-derived species. First, methylfuran was acylated over zeolite H-Beta with acetic anhydride. Second, the acetyl group was reduced to an ethyl group with hydrogen over copper chromite. Third
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service