D149608
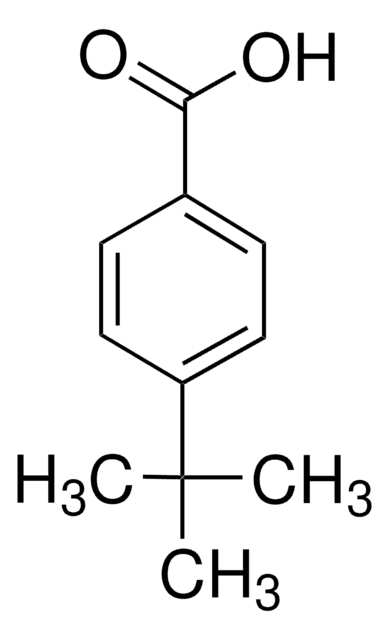
3,5-Dimethylbenzoic acid
99%
Synonym(s):
Mesitylenic acid, sym.-m-Xylylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C6H3CO2H
CAS Number:
Molecular Weight:
150.17
Beilstein:
1072182
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
mp
169-171 °C (lit.)
SMILES string
Cc1cc(C)cc(c1)C(O)=O
InChI
1S/C9H10O2/c1-6-3-7(2)5-8(4-6)9(10)11/h3-5H,1-2H3,(H,10,11)
InChI key
UMVOQQDNEYOJOK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Kostrewski et al.
Toxicology letters, 77(1-3), 259-264 (1995-05-01)
The possibility of evaluating occupational exposure to mesitylene based on the determination of unchanged solvent in capillary blood or 3,5-dimethylbenzoic acid in urine was investigated. The volunteers were exposed to mesitylene in a toxicological chamber (range 10-150 mg/m3). Concentrations of
J Järnberg et al.
International archives of occupational and environmental health, 69(6), 491-497 (1997-01-01)
The aim of this study was to determine the urinary excretion of dimethylhippuric acids (DMHAs) in humans after experimental chamber exposure to trimethylbenzene (TMB) vapor. The DMHAs have been put forward as suitable biomarkers of exposure to products containing TMBs
R B Cain et al.
Biochemical and biophysical research communications, 238(1), 197-201 (1997-09-23)
Here we report that the bacterial catabolism of 4-hydroxy-3,5-dimethylbenzoic acid 1 takes a different course in Rhodococcus rhodochrous N75 and Pseudomonas sp. strain HH35. The former organism accumulates a degradation metabolite of the acid which we isolated and identified as
P Kostrzewski et al.
The Science of the total environment, 199(1-2), 73-81 (1997-06-20)
Trimethylbenzene (TMB) is a component of numerous commercial preparations of organic solvents (Farbasol, Solvesso, Shellsol) used in the chemical, plastics, printing and other industries. TMB is a mixture of three isomers (pseudocumene-1,2,4-TMB; mesitylene-1,3,5-TMB; hemimellitene-1,2,3-TMB). The proportion of individual isomers in
Farooq Ali Shah et al.
Dalton transactions (Cambridge, England : 2003), 44(22), 10467-10478 (2015-05-16)
Three new organotin(iv) carboxylates () of 3,5-dimethylbenzoate, have been synthesized and characterized by elemental analysis, FT-IR, multinuclear NMR ((1)H, (13)C and (119)Sn), mass spectrometry and single crystal X-ray structural analysis. Crystallographic data show that in compounds and , the geometry
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service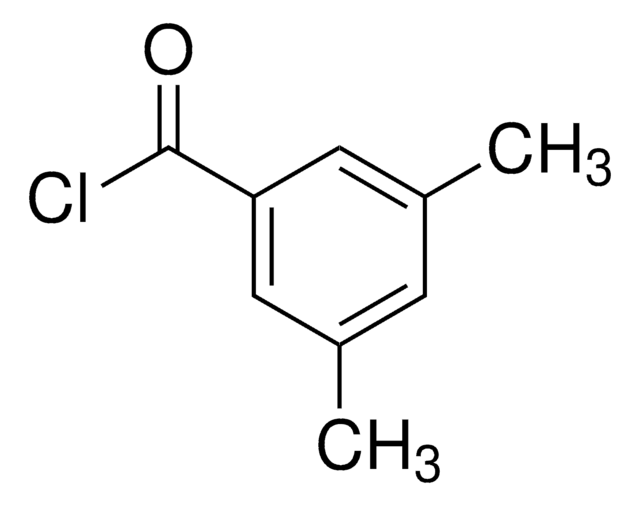
![8-Cyclopropyl-8-azabicyclo[3.2.1]octan-3-one](/deepweb/assets/sigmaaldrich/product/structures/656/354/fa291813-1b40-45b4-8667-d0282a4d9e3c/640/fa291813-1b40-45b4-8667-d0282a4d9e3c.png)