147214
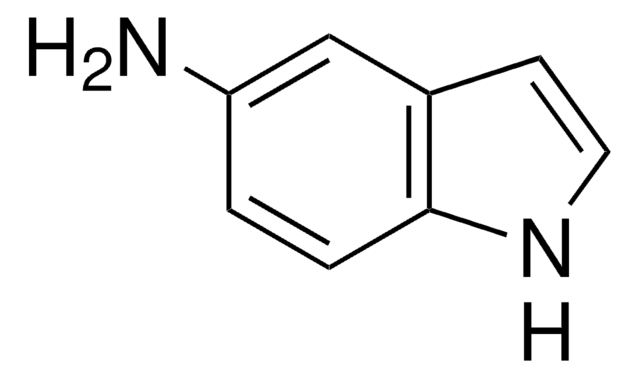
4-Amino-2-nitrophenol
97%
Synonym(s):
4-Hydroxy-3-nitroaniline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H3(NO2)OH
CAS Number:
Molecular Weight:
154.12
Beilstein:
1368435
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
125-127 °C (lit.)
SMILES string
Nc1ccc(O)c(c1)[N+]([O-])=O
InChI
1S/C6H6N2O3/c7-4-1-2-6(9)5(3-4)8(10)11/h1-3,9H,7H2
InChI key
WHODQVWERNSQEO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Amino-2-nitrophenol is the major metabolite of 2,4-dinitrophenol. It is commonly used as semipermanent (nonoxidative) hair colorant and toner in permanent (oxidative) hair dye products.
Application
4-Amino-2-nitrophenol was used in determination of aminonitrophenols in hair dyes by differential pulse voltammetry and HPLC with electrochemical detection.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M A CAMERON
British journal of pharmacology and chemotherapy, 13(1), 25-29 (1958-03-01)
The effect of the mono- and di-nitrophenols and certain related compounds has been determined on the rate of oxygen consumption, the rate of carbon dioxide output and the rectal temperature of the Wistar albino rat.Of the compounds examined, only 2:4-dinitrophenol
Hana Dejmkova et al.
Talanta, 85(5), 2594-2598 (2011-10-04)
Methods for determination of 2-amino-4-nitrophenol and 4-amino-2-nitrophenol, metabolites of 2,4-dinitrophenol, were developed using differential pulse (DP) voltammetry and HPLC with amperometric and spectrophotometric detection. The applicability of these methods was tested by the determination of the analytes in model samples
Determination of Aminonitrophenols in Hair Dyes Using a Carbon Paste Electrode and a Boron-Doped Diamond Film Electrode-A Comparative Study.
Dejmkova H, et al.
International Journal of Electrochemical Science, 6, 3550-3563 (2011)
M M Shahin et al.
Carcinogenesis, 3(7), 809-813 (1982-01-01)
Two samples of 4-amino-2-nitrophenol were tested for mutagenicity in Salmonella typhimurium strains TA1535, TA1537, TA1538, TA98, and TA100. Significant mutagenic activity was detected with a sample of this compound obtained from Aldrich (technical grade (Aldrich), TGA) both in the presence
Christina L Burnett et al.
International journal of toxicology, 28(6 Suppl 2), 217S-251S (2010-01-30)
2-Amino-3-nitrophenol, 2-amino-4-nitrophenol, 2-amino-5-nitrophenol, 4-amino-3-nitrophenol, 4-amino-2-nitrophenol, 2-amino-4-nitrophenol sulfate, 3-nitro-p-hydroxyethylaminophenol, and 4-hydroxypropylamino-3-nitrophenol are substituted aromatic compounds used as semipermanent (nonoxidative) hair colorants and as toners in permanent (oxidative) hair dye products. All ingredients in this group except 2-amino-4-nitrophenol sulfate, 2-amino-5-nitrophenol, and 4-amino-2-nitrophenol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service