Dextran
Read more about
Cas #: 9004-54-0
Structure
Dextran is a polymer of anhydroglucose. It is composed of approximately 95% alpha-D-(1-6) linkages. The remaining a(1-3) linkages account for the branching of dextran.1,2,3 Conflicting data on the branch lengths implies that the average branch length is less than three glucose units.4,5 However, other methods indicate branches of greater than 50 glucose units exist.6,7
Native dextran has been found to have a molecular weight (MW) in the range of 9 million to 500 million.8,9,10 Lower MW dextrans will exhibit slightly less branching4 and have a more narrow range of MW distribution.11 Dextrans with MW greater than 10,000 behave as if they are highly branched. As the MW increases, dextran molecules attain greater symmetry.7,12,13 Dextrans with MW of 2,000 to 10,000 dextran molecules exhibit the properties of an expandable coil.12 At MWs below 2,000 dextran is more rod-like.14
The MW of dextran is measured by one or more of the following methods: low angle laser light scattering15, size exclusion chromatography16, copper-complexation17, and anthrone reagent18 colorometric reducing-end sugar determination and viscosity12.
Specific Rotation: [α]=+199° 11
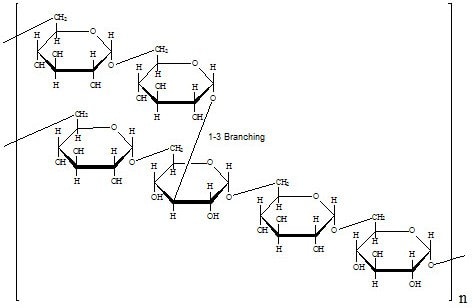
Figure 1.Specific Rotation
Product Information
We offer dextrans which are derived from Leuconostoc mesenteroides, strain B 512. Various MWs are produced by limited hydrolysis and fractionation. Our supplier's exact methods are held proprietary. Fractionation can be accomplished by size exclusion chromatography16 or ethanol fractionation in which the largest MW dextrans precipitate first.19
Preparation Instructions
Dextrans are very water soluble, with the exception of the highest MW dextran, D5501 (MW range = 5 million to 40 million). We test the solubility of dextrans at concentrations generally exceeding 30 mg/mL in water.
Dextrans are also freely soluble in DMSO, formamide, ethylene glycol, and glycerol.11
Neutral-aqueous dextran solutions can be sterilized by autoclaving at 110-115 °C for 30 to 45 minutes.11 Dextran can be hydrolyzed by strong acids at high temperatures. The terminal reducing end group of dextran can be oxidized in alkaline solutions.11
Procedure
Dextran is a high molecular weight, inert, water soluble polymer that has been used in a wide variety of bio-medical applications.
Applications for Unmodified Dextrans:
Plasma Extenders
Dextran solutions have been used as plasma extenders.20
A 10% dextran solution (MW 40,000) exerts a slightly higher colloidal osmotic pressure than plasma proteins.
A 10% dextran (MW 40,000) solution in 0.9% sodium chloride or 5% glucose has reported to be used as a short-term plasma extender for post-operative thrombo-embolic disorders. After infusion, approximately 70% dextran (MW 40,000) is excreted in the urine unchanged after 24 hours. A small amount is eliminated in the feces. The remaining dextran is slowly metabolized to glucose.
A 6% solution of Dextran (MW 70,000) exerts a colloidal osmotic pressure similar to that of plasma proteins. Dextrans with MW greater than 50,000 tend to slowly diffuse across the capillary wall and are slowly metabolized to glucose. Approximately 50% of infused dextran MW 70,000 is excreted unchanged in the urine in 24 hours 20.
Centrifugation / Cell and Organelle Separation
Colloidal solutions containing dextran (MW 250,000) have been used to isolate aggregated platelets21 leukocytes22 and lymphocytes23 in blood by centrifugation.
Dextran (MW 40,000) has been used for the isolation of intact nuclei.24
Protein Precipitation
Dextrans have been used to enhance the precipitation and sensitivity of antibody-antigen complexes in immuno-diffusion applications.
Dextran (MW 80,000) was infused into an immunoelectrophoresis gel at a maximum of 80 mg/mL.25
Dextrans (MW 250,000 and 2,000,000) have also been used in similar applications.26
Inhibition of Platelet Aggregation
At lower concentrations Dextrans (MW 10,000-40,000) have been used to inhibit platelet aggregation.27
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?