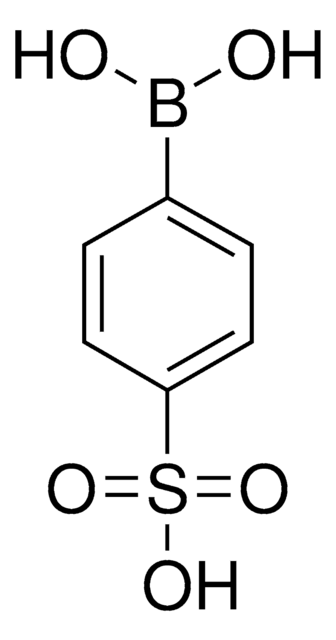
675903
4-(Methanesulfonyl)phenylboronic acid
≥95.0%
Sinónimos:
4-(Methanesulfonyl)benzeneboronic acid, 4-(Methylsulfonyl)phenylboronic acid, 4-Methansulfonylphenylboronic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(H3CSO2)C6H4B(OH)2
Número de CAS:
Peso molecular:
200.02
Número MDL:
Código UNSPSC:
12352103
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
≥95.0%
Formulario
solid
mp
289-293 °C
grupo funcional
sulfone
cadena SMILES
CS(=O)(=O)c1ccc(cc1)B(O)O
InChI
1S/C7H9BO4S/c1-13(11,12)7-4-2-6(3-5-7)8(9)10/h2-5,9-10H,1H3
Clave InChI
VDUKDQTYMWUSAC-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
Contains varying amounts of anhydride
Aplicación
4-(Methanesulfonyl)phenylboronic acid may be used as reagent for:
Reagent used in Preparation of
- sequential Suzuki cross-coupling reactions
- Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids
- directed metalation and regioselective functionalization of 3-bromofuran and related heterocycles
- Barton-Zard pyrrole cyclocondensations and Baeyer-Villiger oxidations
- diplar cycloaddition and palladium-catalyzed cross-coupling processes
- continuous flow Suzuki reactions for odanacatib intermediate synthesis
Reagent used in Preparation of
- diarylaminopyridines as potential anti-malarial agents
- hydropyranopyrazine via chloropyrazinecarboxaldehyde and olefination
- biaryl sulfone derivatives as antagonists of the histamine H3 receptor
- novel kinase inhibitor scaffolds with potential antitumor effects
- Hepatitis C virus inhibition activity of N-hydroxyisoquinoline di
Highly effective boronic acid used in a rhodium-catalyzed asymmetric 1,4-addition to 4-oxobutenamides.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Pamela Kassis et al.
European journal of medicinal chemistry, 46(11), 5416-5434 (2011-09-29)
We here report the synthesis and biological evaluation of new 3-[(2-indolyl)]-5-phenyl-3,5-pyridine, 3-[(2-indolyl)]-5-phenyl-2,4-pyridine and 3-[(2-indolyl)]-5-phenyl-2,6-pyrazine derivatives designed as potential CDK inhibitors. Indoles and phenyls were used to generate several substitutions of the pyridine and pyrazine rings. The synthesis included Stille or
Synthesis of 2,6-disubstituted-7,8-dihydro-6H-pyrano[2,3-b]pyrazines
Li, J-C.; et al.
Tetrahedron Letters, 53, 852-853 (2012)
Jamie L Zigterman et al.
The Journal of organic chemistry, 72(23), 8870-8876 (2007-10-12)
A variety of 4-oxobutenamides 1 were subjected to rhodium-catalyzed conjugate addition with arylboronic acids providing high regio- and enantioselectivity (97:3 to >99:1, >96% ee) and moderate to excellent yields (54-99%). The key to high selectivity is the use of sterically
Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids with TMSCF3 and elemental sulfur.
Chao Chen et al.
Angewandte Chemie (International ed. in English), 51(10), 2492-2495 (2012-01-31)
Identification of biaryl sulfone derivatives as antagonists of the histamine H3 receptor: Discovery of (R)-1-(2-(4'-(3-methoxypropylsulfonyl)biphenyl-4-yl)ethyl)-2-methylpyrrolidine (APD916)
Semple, G.; et al.
Bioorganic & Medicinal Chemistry, 21, 71-75 (2012)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)