494348
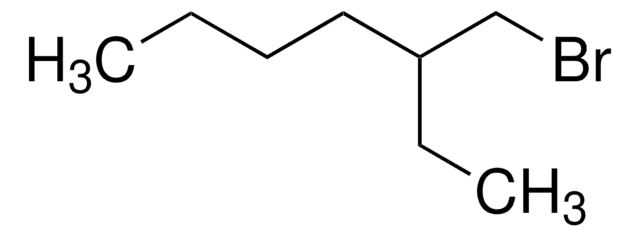
1-Bromo-4-methylpentane
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(CH3)2CH(CH2)3Br
Número de CAS:
Peso molecular:
165.07
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
97%
índice de refracción
n20/D 1.446 (lit.)
bp
146 °C (lit.)
densidad
1.134 g/mL at 25 °C (lit.)
grupo funcional
alkyl halide
bromo
cadena SMILES
CC(C)CCCBr
InChI
1S/C6H13Br/c1-6(2)4-3-5-7/h6H,3-5H2,1-2H3
Clave InChI
XZKFBZOAIGFZSU-UHFFFAOYSA-N
Descripción general
1-Bromo-4-methylpentane can be synthesized by treating 4-methyl-1-pentanol with phosphorous tribromide. The conformational analysis of its liquid-state and solid-state IR and Raman spectra and showed the presence of a mixture of PC,PH, and P′H conformers.
Aplicación
1-Bromo-4-methylpentane may be used in the synthesis of:
- diethyl 2-(4′-methylpentyl)malonate
- 1,3-bis(4-(isopentyloxy)phenyl)urea
- 1,3-bis(4-(isopentyloxy)phenyl)thiourea
- 13-methyl-1-[(tetrahydropyran-2-yl)oxy]tetradec-8-yne
- 15-methyl-1-[(tetrahydropyran-2-yl)oxy]hexadec-10-yne
- 3-[2-isohexyloxy-3-(hydroxymethyl]-5-phenyl-2-isohexyloxybenzyl alcohol
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
73.4 °F
Punto de inflamabilidad (°C)
23 °C
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
E Takano et al.
The Journal of biological chemistry, 275(15), 11010-11016 (2001-02-07)
Early stationary phase culture supernatants of Streptomyces coelicolor A3(2) contained at least four small diffusible signaling molecules that could elicit precocious antibiotic synthesis in the producing strain. The compounds were not detected in exponentially growing cultures. One of these compounds
B Reiter et al.
Journal of chemical ecology, 29(10), 2235-2252 (2003-12-20)
Gas chromatography, coupled gas chromatography-mass spectrometry (electron impact mode and chemical ionization with methane as reactant gas), gas chromatography-infrared spectroscopy, and derivatization techniques were used to identity 53 compounds in the interdigital secretion of the red hartebeest, Alcelaphus buselaphus caama.
Néstor M Carballeira et al.
Chemistry and physics of lipids, 145(1), 37-44 (2006-11-28)
The first total syntheses for the (Z)-15-methyl-10-hexadecenoic acid and the (Z)-13-methyl-8-tetradecenoic acid were accomplished in seven steps and in 31-32% overall yields. The (trimethylsilyl)acetylene was the key reagent in both syntheses. It is proposed that the best synthetic strategy towards
Vibrational analysis of alkyl bromides: Part III. Branched-chain bromides: 1-bromo-3-methylbutane and 1-bromo-4-methylpentane.
Crowder GA and Jalilian MR.
Journal of Molecular Structure, 42, 71-76 (1977)
Maria E Amato et al.
Molecules (Basel, Switzerland), 15(3), 1442-1452 (2010-03-26)
A novel chiral macrocyclic ligand incorporating a chiral salen moiety into a framework containing two biphenyl units was synthesized. Structural properties and conformational aspects of the free ligand and an UO2 complex were studied by using NMR spectroscopy in solution
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico