1.00489
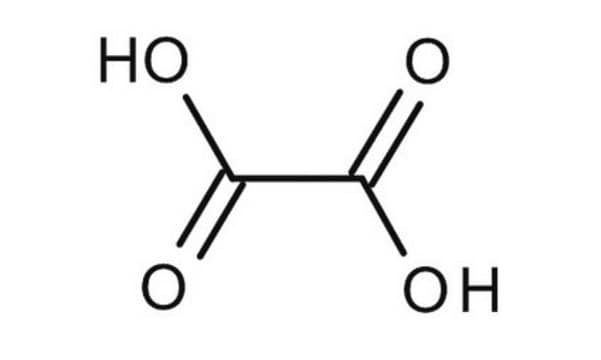
Oxalic acid dihydrate
Suprapur®
Synonyme(s) :
Oxalic acid dihydrate, Ethanedioic acid
About This Item
Produits recommandés
Qualité
for inorganic trace analysis
Niveau de qualité
Pression de vapeur
0.000312 hPa ( 25 °C)
Pureté
≥99.5% (calculated as dihydrate, alkalimetric)
Forme
solid
Puissance
375 mg/kg LD50, oral (Rat)
pH
1.5 (10 g/L in H2O)
Point d'ébullition
149-160 °C/1013 hPa (decomposition)
Pf
98-100 °C
Température de transition
flash point 157 °C (decomposition)
Solubilité
water: >100 g/L at 25 °C
Densité
1.65 g/cm3 at 20 °C
Masse volumique apparente
813 kg/m3
Traces d'anions
chloride (Cl-): ≤5000 ppb
phosphate (PO43-): ≤500 ppb
sulfate (SO42-): ≤2000 ppb
Traces de cations
Ag: ≤10 ppb
Al: ≤20 ppb
As: ≤1.0 ppb
Au: ≤1.0 ppb
Ba: ≤100 ppb
Be: ≤1.0 ppb
Bi: ≤1.0 ppb
Ca: ≤100 ppb
Cd: ≤50 ppb
Co: ≤5 ppb
Cr: ≤10 ppb
Cu: ≤5 ppb
Fe: ≤50 ppb
Ga: ≤1.0 ppb
Ge: ≤1.0 ppb
In: ≤1.0 ppb
K: ≤200 ppb
Li: ≤5 ppb
Mg: ≤20 ppb
Mn: ≤5 ppb
Mo: ≤5 ppb
Na: ≤100 ppb
Ni: ≤10 ppb
Pb: ≤10 ppb
Pt: ≤1.0 ppb
Sb: ≤1.0 ppb
Sn: ≤5 ppb
Sr: ≤100 ppb
Ti: ≤5 ppb
Tl: ≤1.0 ppb
U: ≤1.0 ppb
V: ≤10 ppb
Zn: ≤20 ppb
Température de stockage
2-30°C
InChI
1S/C2H2O4.2H2O/c3-1(4)2(5)6;;/h(H,3,4)(H,5,6);2*1H2
Clé InChI
GEVPUGOOGXGPIO-UHFFFAOYSA-N
Application
- Reaction Atmosphere-Controlled Thermal Conversion of Ferrocene to Hematite and Cementite Nanomaterials-Structural and Spectroscopic Investigations.: This study investigates the conversion of ferrocene to hematite and cementite nanomaterials under controlled thermal conditions. The structural and spectroscopic properties of the resulting materials were analyzed, providing insights into their potential applications in various fields of chemistry and materials science (Kundu et al., 2024).
- Insights into a Co-precursor Driven Solid-State Thermal Reaction of Ferrocene Carboxaldehyde Leading to Hematite Nanomaterial: A Reaction Kinetic Study.: This research focuses on the solid-state thermal reaction of ferrocene carboxaldehyde with oxalic acid dihydrate, leading to hematite nanomaterial. The study provides detailed reaction kinetics and potential applications in catalysis and materials science (Chakraborty et al., 2023).
- Supramolecular Structure of Microwave Treated Bamboo for Production of Lignin-Containing Nanocellulose by Oxalic Acid Dihydrate.: The paper explores the use of oxalic acid dihydrate in the microwave treatment of bamboo to produce lignin-containing nanocellulose. This process enhances the material properties of nanocellulose, offering applications in sustainable materials and bioengineering (Wang et al., 2023).
- Thermo-Mechano-Chemical Deconstruction of Cellulose for Cellulose Nanocrystal Production by Reactive Processing.: This study presents a method for producing cellulose nanocrystals using thermo-mechano-chemical deconstruction with oxalic acid dihydrate. The resulting nanocrystals have potential uses in biocomposites and nanomaterials (Guiao et al., 2022).
- Formation and Structure Evolution of Starch Nanoplatelets by Deep Eutectic Solvent of Choline Chloride/Oxalic Acid Dihydrate Treatment.: This research investigates the formation of starch nanoplatelets using a deep eutectic solvent comprising choline chloride and oxalic acid dihydrate. The study highlights the structural evolution and potential applications in food science and materials engineering (Xiao et al., 2022).
Remarque sur l'analyse
Chloride (Cl): ≤ 5000 ppb
Phosphate (PO₄): ≤ 500 ppb
Sulfate (SO₄): ≤ 2000 ppb
Ag (Silver): ≤ 10 ppb
Al (Aluminium): ≤ 20 ppb
As (Arsenic): ≤ 1.0 ppb
Au (Gold): ≤ 1.0 ppb
Ba (Barium): ≤ 100 ppb
Be (Beryllium): ≤ 1.0 ppb
Bi (Bismuth): ≤ 1.0 ppb
Ca (Calcium): ≤ 100 ppb
Cd (Cadmium): ≤ 50 ppb
Co (Cobalt): ≤ 5 ppb
Cr (Chromium): ≤ 10 ppb
Cu (Copper): ≤ 5 ppb
Fe (Iron): ≤ 50 ppb
Ga (Gallium): ≤ 1.0 ppb
Ge (Germanium): ≤ 1.0 ppb
In (Indium): ≤ 1.0 ppb
K (Potassium): ≤ 200 ppb
Li (Lithium): ≤ 5 ppb
Mg (Magnesium): ≤ 20 ppb
Mn (Manganese): ≤ 5 ppb
Mo (Molybdenum): ≤ 5 ppb
Na (Sodium): ≤ 100 ppb
Ni (Nickel): ≤ 10 ppb
Pb (Lead): ≤ 10 ppb
Pt (Platinum): ≤ 1.0 ppb
Sb (Antimony): ≤ 1.0 ppb
Sn (Tin): ≤ 5 ppb
Sr (Strontium): ≤ 100 ppb
Ti (Titanium): ≤ 5 ppb
Tl (Thallium): ≤ 1.0 ppb
U (Uranium): ≤ 1.0 ppb
V (Vanadium): ≤ 10 ppb
Zn (Zinc): ≤ 20 ppb
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique