RAFT: Choosing the Right Agent to Achieve Controlled Polymerization
The RAFT Process
RAFT or Reversible Addition–Fragmentation chain Transfer is a form of living radical polymerization. RAFT polymerization was discovered at CSIRO in 1998.1 It soon became the focus of intensive research, since the method allows synthetic tailoring of macromolecules with complex architectures including block, graft, comb, and star structures with predetermined molecular weight.2 RAFT polymerization is applicable to a very wide range of monomers under a large number of experimental conditions, including the preparation of water-soluble materials.3
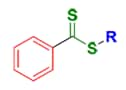
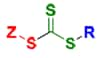
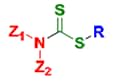
The RAFT process involves conventional free radical polymerization of a substituted monomer in the presence of a suitable chain transfer agent (RAFT agent or CTA). Commonly used RAFT agents include thiocarbonylthio compounds such as dithioesters,1 dithiocarbamates,4,5 trithiocarbonates,6 and xanthates,7 which mediate the polymerization via a reversible chain-transfer process. Use of a proper RAFT agent allows synthesis of polymers with low polydispersity index (PDI) and high functionality (Figure 1).

Figure 1.General comparison of polymers made with traditional radical polymerization against those made using RAFT process.
A RAFT CTA typically has a thiocarbonylthio group (S=C-S) with substituents R and Z that impact the polymerization reaction kinetics and, importantly, the degree of structural control. Initiation of the polymerization reaction is accomplished utilizing conventional thermal, photochemical, or redox methods and it is the proper choice of the RAFT reagents appropriate for a particular monomer and reaction medium that will largely determine success of a RAFT polymerization experiment (Figure 2).

Figure 2.General structure of a RAFT agent; choice of the RAFT agent is critical to obtain polymers with low PDI and controlled architecture.
Dithiobenzoates
|  |
Trithiocarbonates
|  |
Dithiocarbamates
|  |
RAFT Agent to Monomer Compatibility Table
The application of RAFT agents with common monomers used in polymerizations is shown in Table 1. The pluses and minuses represent degree of compatibility between monomer classes and a RAFT agent. For example, Product No. 723037 is very useful in polymerizing styrenes, methacrylates and methacrylamides, shows moderate activity for acrylates and acrylamides but cannot be used on vinyl esters or vinyl amides. This table can be used as a guide for selecting the most appropriate RAFT agent for your needs.

Table 1.A list RAFT agents with their suitability for various monomer types.
References
RAFT agents are sold for research purposes only.
To continue reading please sign in or create an account.
Don't Have An Account?