745146
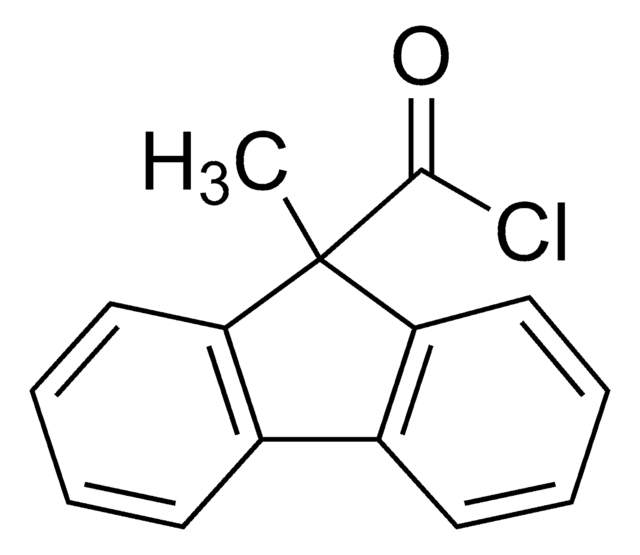
9-Methyl-9H-fluorene-9-carbonyl-13C chloride
≥97.0% (GC)
Synonym(s):
13COgen
About This Item
Recommended Products
isotopic purity
≥97.0%
Assay
≥97.0% (GC)
form
solid
reaction suitability
reaction type: C-C Bond Formation
SMILES string
CC1(c2ccccc2-c3ccccc13)[13C](Cl)=O
InChI
1S/C15H11ClO/c1-15(14(16)17)12-8-4-2-6-10(12)11-7-3-5-9-13(11)15/h2-9H,1H3/i14+1
InChI key
ZQYOOHGEBHBNTP-UJKGMGNHSA-N
Application
For more information please visit: Technology Spotlight, Professor Skrystrup PPP
Use with the COware Platform
Packaging
Linkage
also commonly purchased with this product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Related Content
The Skrydstrup group has developed reagents and glassware for carrying out transition metal catalyzed carbonylations in a simple and safe manner.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service