ALD00629
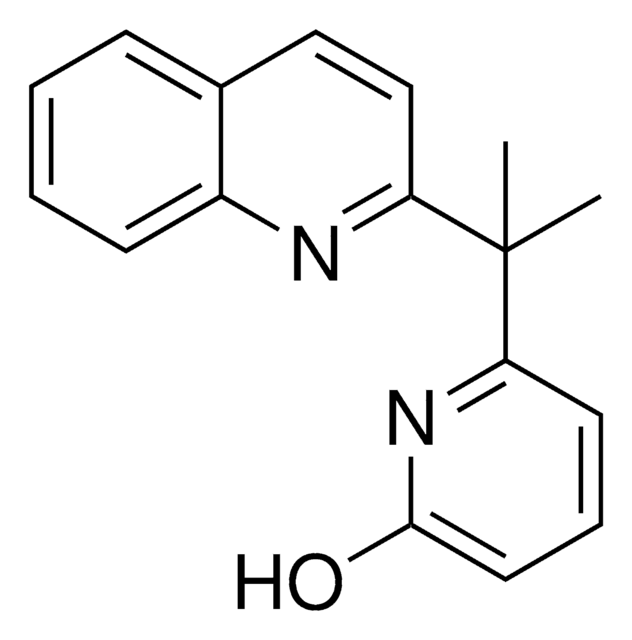
6-(1-Cyclohexyl-1-(5-methylpyridin-2-yl)ethyl)pyridin-2(1H)-one
≥95%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H24N2O
CAS Number:
Molecular Weight:
296.41
UNSPSC Code:
12352100
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥95%
form
powder or chunks
mp
146-149 °C
storage temp.
−20°C
SMILES string
O=C1C=CC=C(N1)C(C2=NC=C(C=C2)C)(C)C3CCCCC3
Application
This ligand was developed by the Yu lab to enable the Pd-catalyzed dehydrogenation of aliphatic carboxylic acids through a typically challenging activation of the β-methylene C-H bond. This method is chemoselective to carboxylic acids even in the presence of other enolizable functional groups, and may also viably utilize molecular oxygen as the terminal oxidant for the transformation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhen Li et al.
Science (New York, N.Y.), 372(6549), 1452-1457 (2021-11-30)
Hydroxylation of aryl carbon-hydrogen bonds with transition metal catalysts has proven challenging when oxygen is used as the oxidant. Here, we report a palladium complex bearing a bidentate pyridine/pyridone ligand that efficiently catalyzes this reaction at ring positions adjacent to
Zhen Wang et al.
Science (New York, N.Y.), 374(6572), 1281-1285 (2021-11-12)
Dehydrogenative transformations of alkyl chains to alkenes through methylene carbon-hydrogen (C–H) activation remain a substantial challenge. We report two classes of pyridine-pyridone ligands that enable divergent dehydrogenation reactions through palladium-catalyzed β-methylene C–H activation of carboxylic acids, leading to the direct
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service