All Photos(1)
About This Item
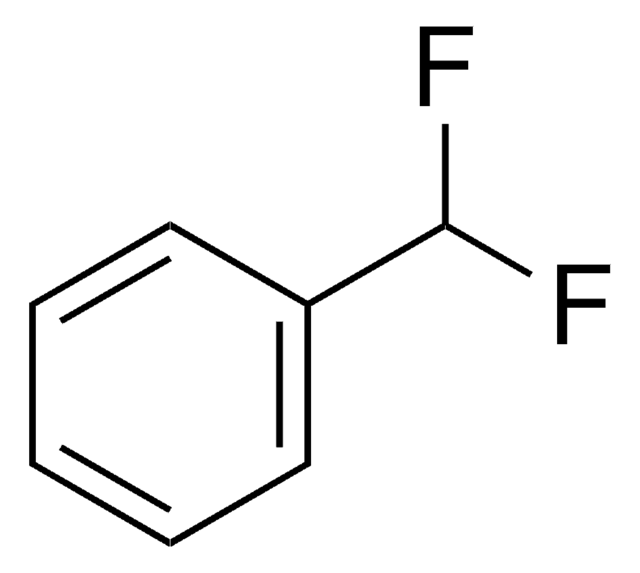
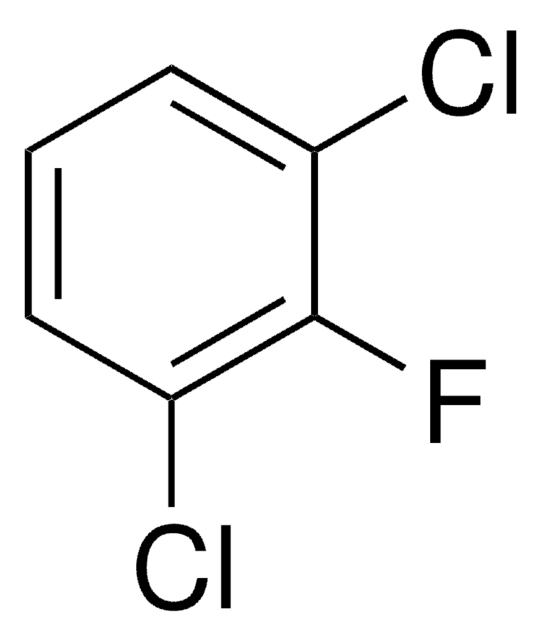
Linear Formula:
CH3C6H3F2
CAS Number:
Molecular Weight:
128.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.449 (lit.)
bp
113-117 °C (lit.)
density
1.12 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc(F)cc1F
InChI
1S/C7H6F2/c1-5-2-3-6(8)4-7(5)9/h2-4H,1H3
InChI key
MPXDAIBTYWGBSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Difluorotoluene was synthesized as a nucleotide analog and was incorporated into DNA and undergoes replication by DNA polymerase enzymes.
Application
2,4-Difluorotoluene was used in the synthesis of new hydrophobic isosteres of pyrimidines and purine nucleosides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
59.0 °F - closed cup
Flash Point(C)
15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shuangluo Xia et al.
Biochemistry, 51(7), 1476-1485 (2012-02-07)
We have recently challenged the widely held view that 2,4-difluorotoluene (dF) is a nonpolar isosteric analogue of the nucleotide dT, incapable of forming hydrogen bonds (HBs). To gain a further understanding for the kinetic preference that favors dAMP insertion opposite
Pradeep S Pallan et al.
Journal of the American Chemical Society, 131(35), 12548-12549 (2009-08-19)
Certain DNA polymerases (pols) were found to efficiently insert A opposite the hydrophobic T isostere 2,4-difluorotoluene (F) and vice versa, resulting in the widely held belief that some pols rely on shape rather than H-bonding for accurate replication. Using X-ray
Adriana Irimia et al.
The Journal of biological chemistry, 282(50), 36421-36433 (2007-10-24)
The 2,4-difluorotoluene (DFT) analog of thymine has been used extensively to probe the relative importance of shape and hydrogen bonding for correct nucleotide insertion by DNA polymerases. As far as high fidelity (A-class) polymerases are concerned, shape is considered by
B D Silverman et al.
Journal of biomolecular structure & dynamics, 16(6), 1169-1175 (1999-08-14)
Molecular moment descriptors of the shape and charge distributions of twenty five nucleoside structures have been examined. The structures include thymidine as well as the difluorotoluene nucleoside analog which has been found to pair efficiently with adenine by polymerase catalysis.
J C Morales et al.
Biochemistry, 39(10), 2626-2632 (2000-03-08)
We describe studies aimed at evaluating the physical factors governing the rate of 3'-end proofreading by the Klenow fragment of E. coli DNA polymerase I. Two nonpolar deoxynucleoside isosteres containing 2,4-difluorotoluene (F) and 4-methylbenzimidazole (Z), which are non-hydrogen-bonding shape mimics
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service