1-tBu-Indenyl Supported Palladium Precatalysts for Cross-Coupling
Introduction
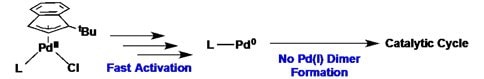
The Hazari Group has developed an improved palladium precatalyst scaffold for a wide range of cross-coupling reactions. This system combines fast activation from Pd(II) to the active monoligated Pd(0) species under mild conditions, with prevention of deleterious formation of any inactive dimeric Pd(I) species. Together, this results in a highly active catalyst system under a variety of conditions. Furthermore, the bench stable precursor, [Pd(1-tBu-Indenyl)Cl]2, (805009) rapidly binds a wide range of phosphine and N-Heterocyclic carbene (NHC) ligands, which allows for facile ligand screening. The ability to generate efficient, palladium precatalysts with either phosphine or NHC ligands is unique.

Advantages
- Air and moisture stable compounds; stable indefinitely
- Effective as a precatalyst for a variety of cross-coupling reactions
- Activation under mild conditions
- Compatible with both phosphine and NHC ligands
Representative Applications
Tetra-ortho Substituted Suzuki-Miyaura Reactions with Pd(1-tBu-Indenyl)(IPr*OMe)(Cl):
Generating tetra-ortho substituted biaryls remains a difficult challenge in cross-coupling. Bulky NHC ligands are used to accomplish this transformation; the 1-tBu-Indenyl system with IPr*OMe as the ancillary ligand generates these products in high yield using low catalyst loadings.

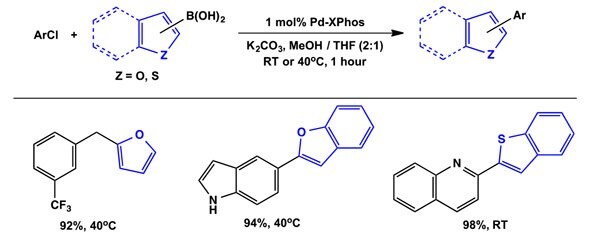
Heteroaryl Suzuki-Miyaura using Pd(1-tBu-Indenyl)(XPhos)(Cl):
Formation of heteroaryl biaryls is an important challenge for current cross-coupling technology. Using XPhos as ancillary ligand, the 1-tBu-Indenyl scaffold effects these transformations at mild conditions, using low catalyst loadings.
Buchwald-Hartwig with Secondary Amines using Pd(1-tBu-Indenyl)(RuPhos)(Cl):
In combination with RuPhos, this precatalyst scaffold effectively catalyzes the reaction between secondary amines and aryl chlorides to generate tertiary amines. Both aliphatic and aryl amines are compatible with this methodology.

Alkyl Trifluoroboronate Salts in Suzuki-Miyaura Reactions using Pd(1-tBu-Indenyl)(P{tBu}3)(Cl):
Forming sp2-sp3 bonds remains a current challenge in cross-coupling. Use of potassium trifluoroboronate salts as nucleophile, in combination with the 1-tBu-Indenyl scaffold, has allowed for vast improvements on established methodologies. In fact, catalyst loadings as low as 1 mol% can be used with this system.
Special thanks to Mr. Patrick Melvin and Prof. Nilay Hazari for contributing this technology spotlight.

References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?