Mycoplasma Clearance Performance of Stericup® and Steritop® Filters
Mycoplasma Contamination in Cell Culture
Mycoplasma, a common microbial contaminant in cell culture systems, poses significant risks to cell lines, biomanufacturing processes, and research outcomes. Effective filtration strategies are essential for preventing mycoplasma contamination. Due to their small size and deformability, mycoplasma can penetrate filters with pore size ratings as low as 0.2 µm. The use of sterilizing 0.1 µm rated filters such as Stericup® systems and Steritop® bottle top units for cell culture media preparation helps support data integrity, cell line stability, and biosafety. While there is currently no industry standard for rating filters for mycoplasma clearance, rigorous filter testing is crucial. We present results using an internally validated Acholeplasma laidlawii challenge test method to assess the mycoplasma clearance performance of 0.1 µm and 0.22 µm sterilizing-grade filters. The A. laidlawii challenge test follows the ASTM® F838-05 Standard Test for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration,1 supporting characterization of membrane filters during development and manufacturing lot release. Our results show that Stericup® and Steritop® filters containing 0.1 µm PES membranes can be used effectively to reduce mycoplasma during cell culture media, buffer, and reagent preparation.
Mycoplasma Clearance
Various filtration techniques are employed for mycoplasma reduction, including membrane filtration and depth filtration. Membrane filtration using specialized membranes with defined pore sizes is widely employed to achieve efficient reduction of mycoplasma particles. Depth filtration, on the other hand, employs fibrous materials to trap and retain contaminants including mycoplasma. The choice of filtration media is crucial for effective mycoplasma reduction. Hydrophilic membranes with pore sizes ranging from 0.1 μm to 0.2 μm demonstrate high retention efficiency, ensuring mycoplasma particles are effectively reduced during filtration processes. Additionally, depth filtration media with proper particle size retention characteristics can further enhance mycoplasma clearance efficiency.
We compared polyvinylidene fluoride (PVDF) and polyethersulfone (PES) membranes with pore size ratings of 0.1 μm and 0.22 μm for reduction of mycoplasma via filtration. To eliminate device impacts and ensure unbiased experimental testing, membranes were extracted from vacuum cups and placed into sterile 47-mm stainless-steel filter holders with coated screen supports. The testing process was modeled to align with small-scale (≤ 1L) filtration devices, simulating real-world filtration volume and conditions at 30 psi (2.07 bar). Samples were evaluated, with size controls run alongside each test to ensure the test organism's size (Acholeplasma laidlawii American Type Culture Collection (ATCC) Rockville, Maryland #23206).
Pre-Use Buffer Sterility Testing
Test membranes were subjected to a pre-use, mycoplasma buffer-wet sterility test to ensure values met or exceeded the specified minimum requirements at or near the stated minimum specification.
Challenge and Buffer Chase
The first buffer sterility check was followed by the A. laidlawii challenge test in modified Hayflick’s media, a proven growth media to maintain consistently yielded high A. laidlawii cell concentrations with small cell size. By using optimizing cultivation methods, the test achieved reproducible and rigorous conditions necessary for evaluating the mycoplasma clearance performance of sterilizing-grade filters. The aim was to assess the filter's ability to retain a minimum challenge of 107 cells of A. laidlawii per cm2 of filter area in ~100 mL volume. Following the challenge delivery, another 100 mL of buffer was used to flow through the filter. The effluents were cultured for seven days and assessed for the presence of A. laidlawii via colony counting on pour plates.
Quality Control
Routine control measures, including testing for mycoplasma presence, were used to check the effectiveness of the filtration process and identify any potential issues.
Mycoplasma Clearance Testing
Membrane Flux
Flow rates were captured using samples from the sterility, challenge, and buffer wash steps. A 100 mL volume was processed at each step, using the defined 13.8 cm2 surface area to calculate flux values. Results are shown in Figure 1. As expected, the 0.1 µm rated filters had lower flux compared to 0.22 µm rated filters. Faster flow rates were achieved using PES membranes. Of the two 0.22 µm PES membranes tested, Stericup® PES membrane filters had ≥ 20% faster flow rates. The low flow control 0.1 µm PVDF had the slowest flow rate, as designed. Challenge and buffer chase samples required longer processing times compared to pre-challenge sterility check samples.

Figure 1.Calculated flux for 0.1 and 0.22 µm PES and PVDF membrane filters. VP = 0.1 µm PES membrane. GP = 0.22 µm PES membrane. GV = 0.22 µm PVDF membrane. VV = 0.1 µm PVDF membrane.
Mycoplasma Retention of Membrane Filters
Samples of the A. laidlawii culture, initial A. laidlawii challenge, and effluent were serially diluted in buffer and enumerated using a pour-plate method with GMA agar.2 Plates were incubated at 37 °C (± 2 °C) with 6% (± 1%) CO2 for seven days. Post-incubation, pour-plates were enumerated and titer was determined.
The following calculations were used to determine Log Reduction Value (LRV):
Equation 1: Total challenge in CFU/filter
Total Challenge = challenge concentration (CFU/mL) x challenge volume (mL/filter)
Equation 2: EFA (effective frontal area challenge in CFU/cm2)

Equation 3: Total passage in CFU/filter*
Total passage = total CFU on the analytical membrane
Equation 4: Log Reduction Value
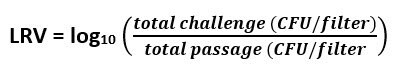
Equation 5: LRV of samples
Challenge/ 100 mL sample (CFU) = challenge concentration (CFU/mL) x challenge volume (100 mL)
Total passage (CFU/100 mL sample) = total CFU on the assay filter
LRV = challenge/100 mL sample (CFU) / total passage (CFU/100 mL sample)
Calculated LRVs shown in Figure 2. Our results indicate that the 0.1 µm rated PES Express® PLUS membrane from Stericup® and Steritop® filters demonstrated superior reduction (LRV ≥ 7) compared to 0.22 µm PVDF and PES membranes (LRV = 2.6 – 3.2). The control low-flow retentive 0.1 µm PVDF membrane had a calculated LRV > 9.1 (fully retentive).

Figure 2.Calculated log reduction values (LRV) for different membrane types. 0.1 μm rated filters demonstrated reduction LRV > 7 (low flow PVDF control, LRV of ≥ 9.1; PES, LRV ≥ 7.5) of bacteria. All tested 0.22 μm rated PES and PVDF filters reduced A. laidlawii with log reduction values between 2.6 and 3.2. Standard deviation is indicated by error bars. VP = 0.1 μm PES membrane. GP = 0.22 μm PES membrane. GV = 0.22 μm PVDF membrane. VV = 0.1 μm PVDF membrane.
Stericup® and Steritop® Filters for Mycoplasma Clearance
Filtration plays a critical role in maintaining the purity and safety of biological products in various research, development, and manufacturing processes. By employing microfiltration strategies into cell culture workflows, researchers can mitigate the risks associated with mycoplasma contamination and ensure the integrity and quality of their biological materials. The application of the ASTM® F838-05 Standard Test for Determining Bacterial Retention of Membrane Filters provides a recognized framework for characterizing membrane filters. Performance indicates that these filters can be used to mitigate the risk of mycoplasma contamination in upstream cell culture processes. Our results show that Stericup® and Steritop® filters containing 0.1 µm PES membranes can be used effectively to reduce mycoplasma during cell culture media and buffer preparation. Understanding filtration capabilities and implementing appropriate filtration products into cell culture workflows is essential for effective mycoplasma reduction.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?