Lopinavir HPLC Assay Method
Anita Piper
Introduction
This paper illustrates how it is possible to set-up the assay method for Lopinavir testing following the current European pharmacopeia guidelines (10.2). The monograph assay method calls for a column with l = 0.25 m, Ø = 4.6 mm end-capped octadecylsilyl silica gel for chromatography with 4 µm particle size. No particular HPLC column is referenced in the EP knowledge database for assay method, and the method is of isocratic nature.
This gives a chance to replace the monograph column geometry/particle size with a shorter and faster alternative column (up to 70% reduction in length) packed with smaller particles (up to 50% reduction). This can save valuable time, and at the same time you can benefit from improved separation efficiency, which typically translates into better method performance and sensitivity. In this study, the limit of detection (LOD) is better than 1 ppm using HPLC-UV detection.
Figure 1. Lopinavir

Figure 2. Reference Solution (a)
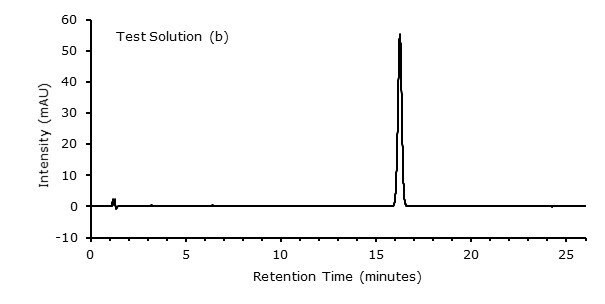
Figure 3. Test Solution (b)
Specificity: Inject reference solution (a) and determine the retention time and the content of desired analyte. |
|---|
Standard Repeatability (25 ppm) |
|---|
LOD & LOQ |
|---|
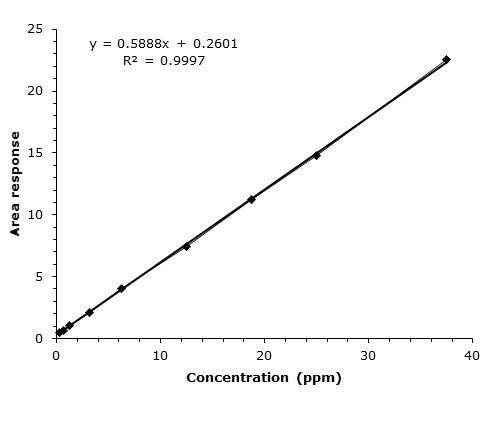
Figure 4. Concentration
Conclusion
In this study in reference to European Pharmacopeia 10 Guidelines, a shorter and faster Fused-Core® (Superficially Porous Particle, SPP) column was evaluated for an assay method for Lopinavir, achieving a limit of detection (LOD) of better than 1 ppm using HPLC-UV detection.
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?