LC/MS/MS of Digoxin and Digitoxin in Serum or Plasma on Titan C18 after SPE with HybridSPE®-Phospholipid, Minimization of Matrix Effects
Materialien
SPE-Röhrchen oder -Platte
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
sample/matrix
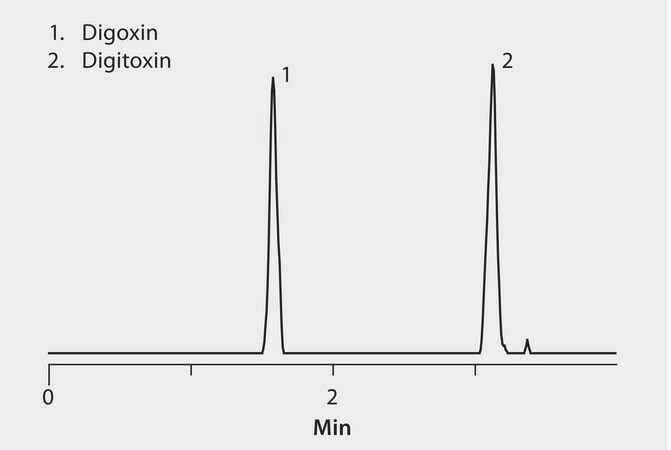
rat plasma or human serum spiked at 0.05 ng/mL with digitoxin and digoxin
SPE well plate
HybridSPE-PLus 96-well plate, 50 mg/well (575659-U)
sample addition
100 μL spiked rat plasma followed by 300 μL 1% formic acid in acetonitrile
elution
apply vacuum at 10 in Hg for 4 min
column
Titan C18, 10 cm x 2.1 mm I.D., 1.9 μm particles (577124-U)
mobile phase
10 mM ammonium formate in methanol:water (80:20)
flow rate
0.2 mL/min
pressure
4550 psi (314 bar)
detector
MS, ESI(+), MRM 798.5/651.5 (digoxin), 782.5/635.5 or 243.2 (digtoxin)
injection
2 μL
Beschreibung
Hinweis zur Analyse
The cardiac glycosides digitoxin and digoxin are widely prescribed for treating congestive heart failure. Immunoassay-based methods are not able to effectively differentiate these two compounds due to their similar chemical structures. This application demonstrates a rapid, selective and sensitive LC/MS/MS method for determination of digitoxin and digoxin in plasma with a simple sample cleanup technique using HybridSPE-PLus for simultaneous removal of proteins and phospholipids and a Titan C18 column for rapid and efficient UHPLC separation. The highest grade LC-MS solvents were used to supply low background interference and low particulate contaminants for robust, trouble-free operation. Cerilliant CRMs provided reliable quantification.
Rechtliche Hinweise
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany
null