The newest air, moisture, and thermally stable crossing-coupling complexes
Introduction to Buchwald Precatalyst Development
Over the last decade, the Buchwald group has developed a series of highly active and versatile palladium precatalysts from the corresponding family of biarylphosphine ligands. These compounds are commonly referred to as Buchwald precatalysts and ligands and have become a valuable set of tools for use in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds.1,2,3,4 The ligands are electron-rich and highly tunable to provide catalyst systems with a diverse scope, high stability, and reactivity.
Current generation Buchwald precatalysts are air, moisture, and thermally-stable and display good solubility in common organic solvents. The use of these precatalysts in cross-coupling reactions typically allows the researcher to use lower catalyst loading and results in shorter reaction times. Additionally, their use ensures the efficient and rapid generation of the active catalytic species, generally without reducing agents, and allows one to accurately control the ligand:palladium ratio. The unique features of the precatalysts have led to the discovery of new methods that would not otherwise be feasible using traditional Pd sources.1,2

Figure 1.Four generations of Buchwald’s precatalysts with continual improvements to their ease of activation.
The first generation (G1) Buchwald precatalysts allowed easier generation of active Pd(0), requiring merely a deprotonation with base. The resulting catalyst is very active, even at temperatures down to -40 °C. Second generation (G2) substituted the phenethylamine based backbone of the (G1) complex with a biphenyl-based ligand. This improvement allowed the researcher to create the active Pd species at room temperature with weak phosphate or carbonate bases. These catalysts prove remarkably adept at achieving a variety of Suzuki-Miyaura couplings among other cross-coupling reactions. However, the earlier generation catalysts suffered from significant limitations either in their synthesis or in the scope of their applications. Replacing the chloride in the precatalysts with a more electron withdrawing, non-coordinating methanesulfonate led to third generation (G3) precatalysts, which are the most versatile and are able to accommodate very bulky ligands such as the BrettPhos (718742) family. Furthermore, these catalysts show remarkably long life in solution.

Figure 2.Buchwald G3 precatalyst
During the activation, deprotection of G3 precatalyst gives a Pd−amido complex which then reductively eliminates to yield active LPd(0), a methanesulfonate salt and carbazole. In some rare cases, the carbazole can inhibit the catalysis by consuming valuable reaction feedstock and also complicating the workup/purifiation. Additionally, trace amounts of residual primary aminobiphenyls can pose potential health risks in pharmaceutical applications. To circumvent these issues, the Buchwald group has modified the G3 catalyst by methylating the amino group of the biphenyl backbone. These fourth generation (G4) precatalysts yield exhibit higher solubilities in cross-coupling reactions while maintaining excellent catalytic activity.

Figure 3.Buchwald G4 precatalyst
Advantages of G3 and G4 Buchwald Precatalysts Over Earlier Generations
- Quantitative generation of the active Pd(0) catalyst
- Accommodates a broader ligand scope
- G3 catalysts are compatible with the BrettPhos family, and extremely bulky t-Bu phosphines
- Enhanced stability in solution, compared to G1 and G2 precatalysts
- G4 precatalysts generate less intrusive N-methylcarbazole byproduct
Representative Coupling Applications and Reaction Scope
G3 and G4 precatalysts are both effective when employed in the Suzuki–Miyaura coupling of unstable boronic acids that are commonly prone to protodeboronation. The success of this coupling process is dependent on very rapid activation of the precatalyst in conjunction with a high level of catalytic activity. XPhos Pd G3 precatalyst (763381) couples unstable boronic acids with electron-rich, sterically hindered, and heteroaryl chlorides under mild conditions (rt to 40 °C) with short reaction times (30 minutes) and in high yields. Similarly, the XPhos Pd G4 precatalyst (804274) successfully provides the biaryls in very high yields.

Scheme 1.XPhos Pd G3 and G4 precatalyst mediated Suzuki-Miyaura coupling reaction
Highly active XantPhos Pd G4 (900329) precatalyst is suitable for low-temperature aminocarbonylations of (hetero)aryl bromides to synthesize challenging products in very good yields with only a slight excess of CO.

Scheme 2.Aminocarbonylations of (hetero)aryl bromides using XantPhos Pd G4.
EPhos Pd G4 (901220) is the precatalyst of choice for palladium-catalyzed C-N cross-coupling between primary amines and aryl halides in the synthesis of functionalized 4-arylaminothiazoles and 2-arylaminooxazoles.

Scheme 3.EPhos Pd G4 catalyzed cross-couplings of 2-aminooxazoles with aryl halides.
VPhos Pd G4 (901218) along with octanoic acid/sodium octanoate surfactant system enables rapid synthesis of a wide spectrum of alkylated scaffolds from alkyl zinc reagents. It is generally used to catalyze Lipshutz–Negishi cross coupling reactions of alkyl halides with aryl electrophiles.

Scheme 4.VPhos Pd G4 catalyzed cross-coupling of alkyl halides with aryl electrophiles.
(t-Bu)PhCPhos Pd G3 (900534) is a hybrid (alkyl)aryl ligated precatalyst, usually preferred for the arylation of highly hindered α,α,α-trisubstituted primary amines with various (hetero)aryl chlorides and bromides.
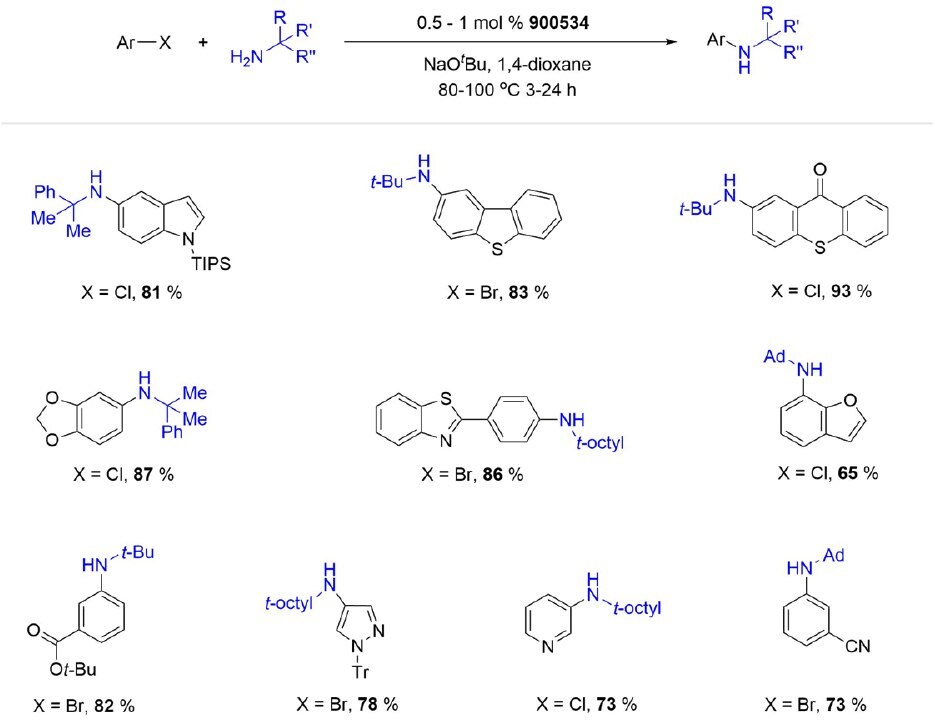
Scheme 5.(tBu)PhCPhos Pd G3 catalyzed arylation of hindered primary amines
The RockPhos Pd G3 precatalyst (773905) has demonstrated utility in C–O bond formation, coupling primary aliphatic alcohols with aryl halides in good to excellent yields.

Scheme 6.RockPhos Pd G3 catalyzed C-O cross-coupling of aryl halides with primary alcohols.

Scheme 7.BrettPhos Pd G3 and G4 catalyzed arylation of primary and secondary amines.
The even-bulkier tBuBrettPhos Pd G3 precatalyst (745979) is very efficient in the arylation of primary amides and tolerant of some functional diversity. The catalyst readily arylates the amide but resists the competitive arylation of the indole and unprotected alcohol group in the explored substrates. Additionally, this versatile palladium precatalyst is an excellent choice for C-O cross-coupling of aryl halides with a broad spectrum of primary alcohols, methanol, and ethanol. Interestingly, it also allows the cross-coupling of both potassium and cesium hydroxides with (hetero)aryl halides to afford hydroxylated heteroarenes in good to excellent yields.

Scheme 8.a. tBuBrettPhos Pd G3 catalyzed arylation of amides

Scheme 8.b.tBuBrettPhos Pd G3 catalyzed aminocarbonylation of (hetero)aryl bromides

Scheme 8.c.tBuBrettPhos Pd G3 catalyzed hydroxylation of aryl and heteroaryl halides

Scheme 8.d.tBuBrettPhos Pd G3 catalyzed C−O cross-coupling of primary alcohols.
AdBrettPhos Pd G3 (776106) with a bulky adamantane moiety is highly efficient precatalyst for the selective amination of a broad range of five-membered heteroaryl halides to synthesize various amino-substituted pyrazoles, benzothiazoles, indazoles and imidazoles.

Scheme 9.AdBrettPhos Pd G3 catalyzed arylation of ammonia with five-membered heteroaryl halides.
References
Abyste mohli pokračovat ve čtení, přihlaste se nebo vytvořte účet.
Nemáte účet?