46192
Paraoxon-methyl
PESTANAL®, analytical standard
Synonyma:
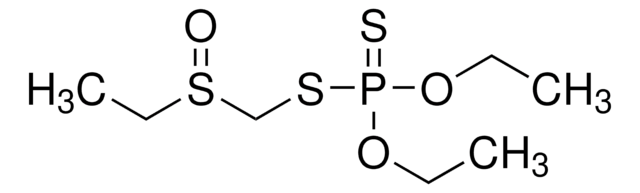
O,O-Dimethyl O-(4-nitrophenyl) phosphate
About This Item
Doporučené produkty
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
NMR: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.523
mp
0-4 °C
suitability
passes test for identity (NMR)
application(s)
agriculture
environmental
format
neat
storage temp.
2-8°C
SMILES string
COP(=O)(OC)Oc1ccc(cc1)[N+]([O-])=O
InChI
1S/C8H10NO6P/c1-13-16(12,14-2)15-8-5-3-7(4-6-8)9(10)11/h3-6H,1-2H3
InChI key
BAFQDKPJKOLXFZ-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
Recommended products
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 1 Inhalation - Acute Tox. 1 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 1
target_organs
Nervous system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
212.0 °F - closed cup
flash_point_c
100 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.