SML2022
UCSF924
≥98% (HPLC)
Synonym(s):
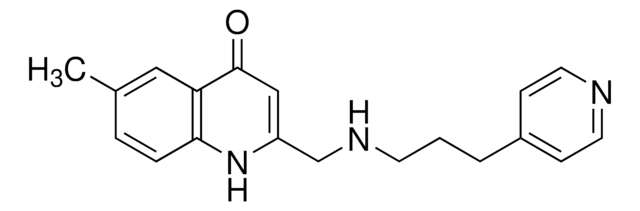
6-Methyl-2-((3-phenoxypropylamino)methyl)quinolin-4(1H)-one, ZINC000091726127
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H22N2O2
CAS Number:
Molecular Weight:
322.40
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
−20°C
SMILES string
CC1=CC=C(NC(CNCCCOC2=CC=CC=C2)=CC3=O)C3=C1
Biochem/physiol Actions
UCSF924 (Compound 9-6-24; ZINC000091726127) is a selective, high-affinity dopamine D4 receptor (DRD4) partial agonist (Ki = 3 nM for human D4) with a 7.4-fold bias toward arrestin recruitment over Gαi (Gαi/0; Gi/G0) signaling activation with respect to quinpirole. UCSF924 exhibits no detectable affinity for D2, D3 or the F261V/L328F D4 mutant and no agonist activity toward a panel of 320 nonolfactory GPCRs even at a high concentration of 1 μM. The UCSF924 structure analog UCSF924NC (Comound 9-6-16; ZINC000091707446) is the recommended negative control compound with a 1/2500-fold reduced D4 affinity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sheng Wang et al.
Science (New York, N.Y.), 358(6361), 381-386 (2017-10-21)
Dopamine receptors are implicated in the pathogenesis and treatment of nearly every neuropsychiatric disorder. Although thousands of drugs interact with these receptors, our molecular understanding of dopaminergic drug selectivity and design remains clouded. To illuminate dopamine receptor structure, function, and
Fiorenza Stagni et al.
Neurobiology of disease, 103, 11-23 (2017-04-01)
Neurogenesis impairment is considered a major determinant of the intellectual disability that characterizes Down syndrome (DS), a genetic condition caused by triplication of chromosome 21. Previous evidence obtained in the Ts65Dn mouse model of DS showed that the triplicated gene
Gary Probst et al.
Journal of medicinal chemistry, 56(13), 5261-5274 (2013-05-30)
Herein, we describe our strategy to design metabolically stable γ-secretase inhibitors which are selective for inhibition of Aβ generation over Notch. We highlight our synthetic strategy to incorporate diversity and chirality. Compounds 30 (ELND006) and 34 (ELND007) both entered human
Inger Lauritzen et al.
Acta neuropathologica, 132(2), 257-276 (2016-05-04)
Endosomal-autophagic-lysosomal (EAL) dysfunction is an early and prominent neuropathological feature of Alzheimers's disease, yet the exact molecular mechanisms contributing to this pathology remain undefined. By combined biochemical, immunohistochemical and ultrastructural approaches, we demonstrate a link between EAL pathology and the
Xiaocong M Ye et al.
Bioorganic & medicinal chemistry letters, 23(4), 996-1000 (2013-01-15)
Structure-activity relationship (SAR) of a novel, potent and metabolically stable series of benzo [3.2.1] bicyclic sulfonamide-pyrazoles as γ-secretase inhibitors are described. Compounds that are efficacious in reducing the cortical Aβx-40 levels in FVB mice via oral dose, as well as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service