902136
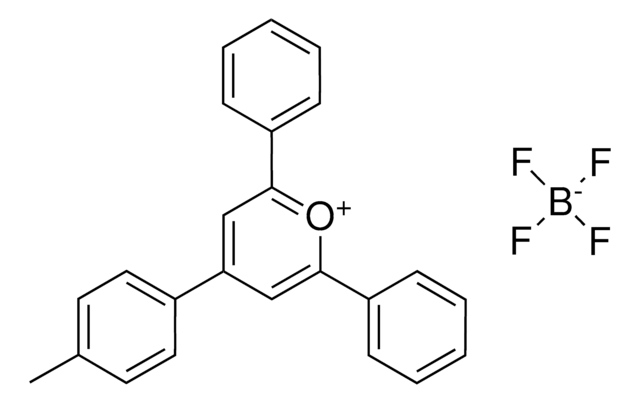
2,4,6-Tri-(4-fluorophenyl)pyrylium tetrafluoroborate
≥95%
Synonym(s):
Triphenylpyrylium photosensitizer, [T(p-F)PPT
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C23H14BF7O
CAS Number:
Molecular Weight:
450.16
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder
reaction suitability
reagent type: catalyst
reaction type: Photocatalysis
mp
242-245 °C
photocatalyst activation
465 nm
Application
Triarylpyrylium salt used as a photosensitizer in photocatalysis and material science.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photocatalytic Cross-Dehydrogenative Amination Reactions between Phenols and Diarylamines.
Zhao Y, et al.
ACS Catalysis, 7 (4), 2446-2451 (2017)
Antonio Franconetti et al.
Physical chemistry chemical physics : PCCP, 16(34), 18442-18453 (2014-07-30)
Noncovalent interactions of anions with electron-deficient aromatic rings that have been studied so far involve non-heteroaromatic or nitrogen-based heteroaromatic systems. Here we report the first case of an organic oxygenated aromatic system, in particular the tri-aryl-pyrylium tetrafluoroborate system, for which
Kuai Wang et al.
Organic letters, 19(8), 1958-1961 (2017-04-04)
A highly regioselective [2 + 2 + 2] cyclization of aromatic alkynes with nitriles is developed for the preparation of 2,3,6-trisubstituted pyridines under visible-light irradiation using a pyrylium salt as the photoredox catalyst. This cycloaddition is achieved through a photooxidative
Ji Young Cho et al.
The Journal of organic chemistry, 83(2), 805-811 (2017-12-14)
Dibenzofurans are naturally occurring molecules that have received considerable attention for a variety of practical applications, such as in pharmaceuticals and electronic materials. Herein, an efficient and eco-friendly method for the synthesis of dibenzofuran derivatives via intramolecular C-O bond formation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Methyl 2-(9-bromo-2,3-dioxo-2,3,6,7-tetrahydro-1H,5H-pyrido[1,2,3-de]quinoxalin-5-yl)acetate ≥90%](/deepweb/assets/sigmaaldrich/product/structures/955/217/e6cb560d-1177-4dc4-b686-7c790e13f1f4/640/e6cb560d-1177-4dc4-b686-7c790e13f1f4.png)






