Analysis of Polar Compounds with Ion Pair Reagents
Hansjörg Tinner
AnalytiX Volume 6 Article 4
The proper selection and quality of reagent, its purity, and high carbon load HPLC columns are critical to the success of HPLC separations employing IPC
Because reversed-phase HPLC is primarily dependent on hydrophobic interactions between the stationary phase and analyte, ion pairing is occasionally necessary to obtain sufficient retention of polar, ionizable compounds. Although there is debate over the precise mechanism, ion pair reagents ultimately enhance retention by increasing the amount of time the analyte spends in the stationary phase over the mobile phase. In addition to enhancing retention, ion pair reagents can also be used to change the selectivity, improve analyte solubility and suppress unwanted interactions, like adsorptive processes in silica-based particles.
The choice of ion pair reagent depends on the analyte, mobile phase conditions, detection method, and how much retention increase is desired. When using ion pairing, the mobile phase pH should allow at least partial ionization of the analyte molecules. Most conventional ion pair reagents are strong acids or bases, so pH does not influence their ionization state within the normal HPLC operating pH range. Counterions should be compatible with the mobile phase and analytes.
The quality of ion pairing reagents is also a very important consideration. Impure reagents can cause noisy or drifting baselines and irreproducibility. Insoluble matter can foul columns and sensitive instrument components. For many years, our ion pair reagents have represented the highest quality in a wide range of available chemistries.
They are subject to extensive application-specific QC testing, including:
- Filter test
- UV-absorption test
- Test for the absence of redox traces with a cyclic voltammogram
- HPLC gradient test
In this article, we will demonstrate the suitability of our ion pair reagents for RP-HPLC of three classes of polar, small molecule analytes: tetracyclines, vitamin B and phosphorylated compounds. Since the analytes were both polar and silanophilic, a Supelco Ascentis C18 HPLC column, which has a high carbon load of 25% for increased retention capability and an inert surface for symmetrical peaks, was chosen to complement the benefits of our brand ion pair reagents.
Tetracyclines
Tetracyclines are broad-spectrum antibiotics prepared from the cultures of certain Streptomyces species. Their analysis by RP-HPLC is difficult because of the presence of polar hydroxyl, carbonyl, and amine groups (Figure 1).
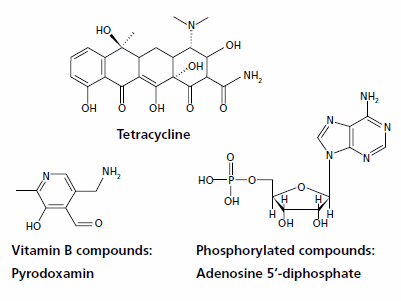
Figure 1.Representative structures
Without ion pair additives, retention and resolution is inadequate. However, by adding an anionic ion pair additive, in this case 1-octane sulfonic acid, retention is significantly increased and resolution is greatly improved (Figure 2). The pH of the mobile phase was important; above pH 5, chlortetracycline and doxycycline could not been separated and fronting was observed. Alkylsulfonate derivatives are in general applied under strong acidic conditions, which is in line with the obtained results. However, tetracyclines, particularly chlortetracycline, are not stable below pH 2 as they transform into dehydro derivates. However, this degradation did not occur during the short duration of the HPLC analysis reported here.
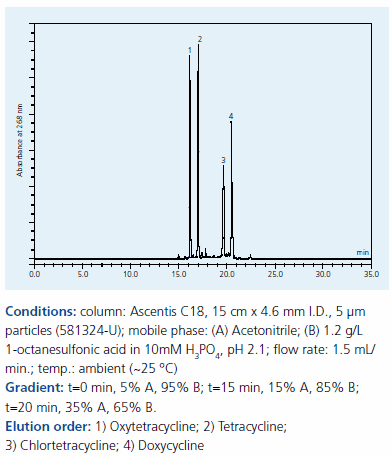
Figure 2.Separation of tetracylines on Supelco Ascentis C18 by IPC with our 1-octane sulfonic acid
Vitamin B compounds
These low molecular weight, polar N-containing compounds (Figure 1) are ionized below pH 7 with concomitant poor RP retention and peak shape. As with the tetracyclines, 1-octane sulfonic acid was added to the mobile phase to enhance retention (Figure 3). The pH of 2.1 ensured ionization of the amine group. Note the excellent peak shape provided by the Ascentis C18 column.
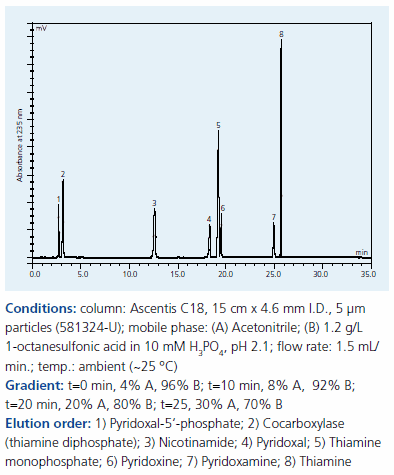
Figure 3.Separation of vitamin B compounds on Supelco Ascentis C18 by IPC with our 1-octane sulfonic acid
Phosphorylated compounds
Phosphorylated compounds (Figure 1) are prevalent in biochemical analyses. To achieve RP retention and separation, tetrabutyl ammonium bisulfate at pH 7.0, which interacts with the phosphate group, was added to the mobile phase (Figure 4). Because pyridoxal-5’- phosphate, thiamine monophosphate and cocarboxylase contain both amine and phosphate groups, they can accommodate both anionic and cationic ion pair reagents. Note that Figures 3 and 4, both of which contain these compounds, show a peak order reversal with the two different reagents. This indicates that the retention mechanism is influenced by more than just compound polarity. It also reinforces the fact that the choice in IPC reagent can be leveraged in order to optimize the separation.
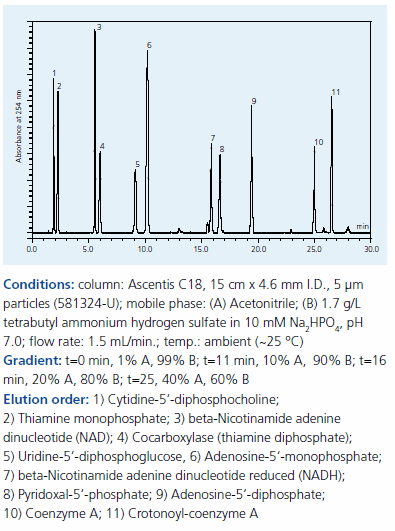
Figure 4.Separation of phosphorylated compounds on Supelco Ascentis C18 by IPC with our tetrabutylammonium sulfate
Conclusion
The combination of our ion pair reagents and Supelco Ascentis columns is an excellent solution for the analysis of highly polar, silanophilic compounds by RP-HPLC. The combination leverages the power of RP-HPLC to differentiate between closely-related molecules and the power of ion pairing to enhance retention and selectivity. Using the ion pairing method described here, difficult to analyze polar compounds were successfully separated with adequate retention and resolution, but without tailing, fronting or co-elution.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?