Data Analytics Built for Biotech
Visualizations, Analytics, and Process Monitoring for
Data-Driven Decisions
Without a process data management tool, bioprocess engineers and scientists routinely spend up to 80% of their time finding, cleaning, and organizing data, and only 20% of their time on actual data analysis. With the right data management tool, these numbers can be reversed, and engineers and scientist can better spend their time gaining process insights through data analysis.
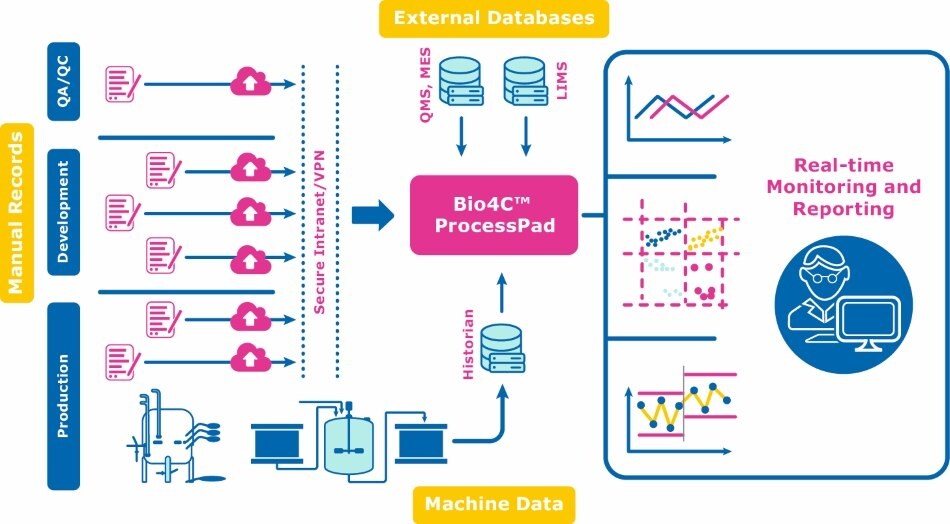
Bio4C ProcessPad™ software is a data collection, visualization, and analytics platform that enables bioprocess monitoring, reporting, investigations, and continued process verification (CPV).
1. Easily Collect Data
2. Automatically Aggregate Data
3. Visualize and Analyze Data
Continued Process Verification (CPV)
So that biopharmaceutical manufacturers can ensure their processes are in a state of control and will continue to produce products of the expected strength, quality, and purity, the FDA and the EMA have made continued process verification (CPV) or ongoing process verification a regulatory expectation.
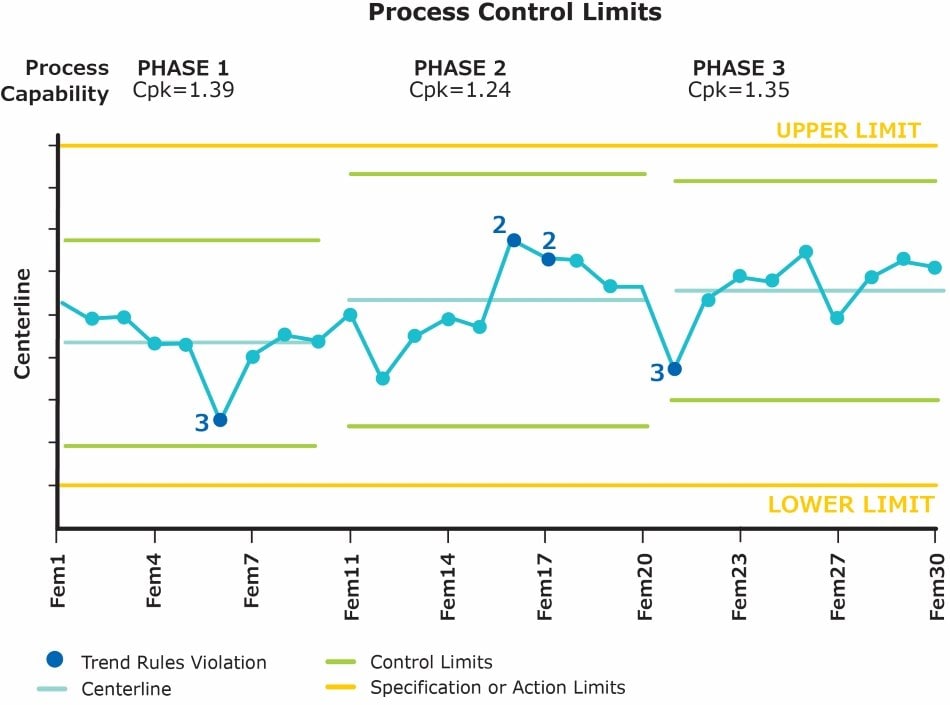
CPV can be a tedious task. It involves collecting data from all the batches executed in a timeframe and trending them with statistical control limits, calculation of process capabilities, adding any statistical rule violations, and more. To learn more about CPV, read our article.
Bio4C ProcessPad™ Automates and Simplifies Many Steps Involved in CPV Including:
- Prediction of data distribution
- Automatic capability analysis (Cpk and Ppk calculations)
- Monitoring parameters against set limits
- Control limit management
- Automatic calculation of statistical trend rule violation
- CPV reporting
Reporting - Run Reports With Just One Click
Bio4C ProcessPad™ removes most of the manual tasks associated with reporting and, as a single source of truth, can automatically generate reports on any data available within the software.
Bio4C ProcessPad™ Software Has the Following Built-In Reports:
- Process summary reports
- Annual product quality reports (APQR)
- Manufacturing campaign reports
- Batch summary and run excursion reports
- Plant equipment utilization report
- Ad hoc reports in support of root cause analysis
Reports can be generated on-demand or scheduled on a daily, weekly, or monthly basis and to ensure they meet your specific process needs, you can also create and save templates for custom reports.
Data Analytics and Visualizations for Process Monitoring and Investigations
Using the right analysis type can bring greater process insights, enable process monitoring, and yield a faster resolution of investigations and CAPA implementation.
Bio4C ProcessPad™ Software Has Out-Of-The-Box Data Analysis Tools Such As:
Lot Tree View – provides information about the inputs and ingredients (e.g., raw materials, product intermediates) for each stage and process of a batch; if you have trouble with a batch, you can see if there were issues with other batches with the same media or input lots
Timelines – gives a holistic view of all batches that ran within a selected time period
Profile Overlay – time series data can be viewed in a profile overlay (e.g., VCD readings over 12 days); compare time profiles of multiple batches for one or more parameters
Control Charts – give insights into batch-to-batch variability or can be used as part of CPV
Relate Parameters – visualizations to study bivariate and multivariate relationships between different batch parameters
PCA (Principal Component Analysis) – enables quick identification of outlier batches within hundreds of CPPs and CQAs and helps determine which process attributes are contributing to process variability

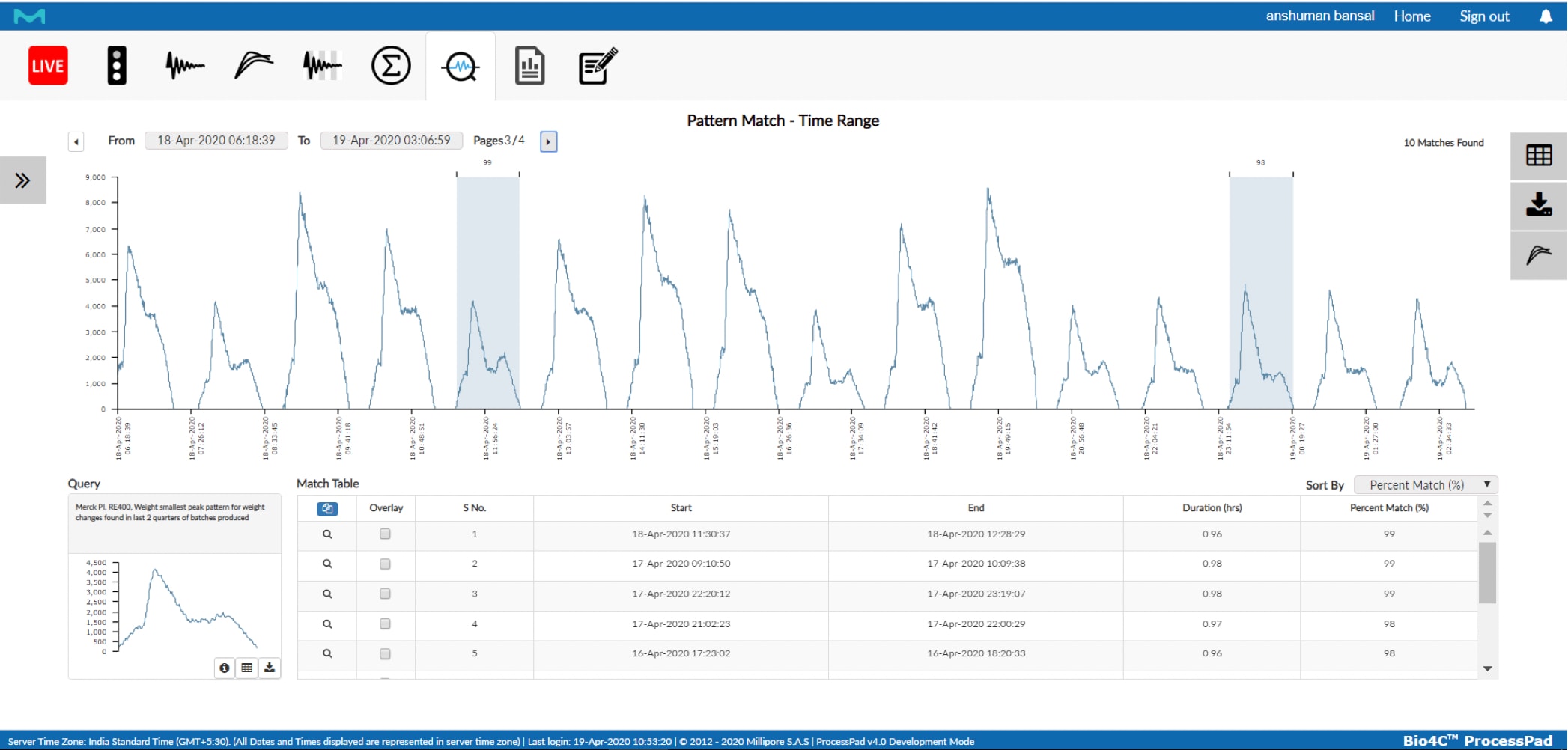
Machine Data Analytics
Analyzing and visualizing streaming data from process equipment, sensors, actuators, etc. can give better process understanding and minimize the time from issue discovery to root cause investigations.
Bio4C ProcessPad™ Machine Data Analytics and Visualizations Supply:
- Live plant, machine, or batch status
- Overall equipment efficiency estimations
- Overlay current and historical batch profiles for comparison and benchmarking
- Correlate streaming parameters
- Align multiple batch events to compare and troubleshoot batch differences
- Search historical time series data for matching anomaly
Related Resources
- Continued Process Verification for Quality
Continuous process verification maintains drug product quality and regulatory compliance throughout production.
- Improving Bioprocess Monitoring and Control with Multivariate Data Analysis
Multivariate data analysis (MVDA) makes possible a proactive, real-time approach to monitoring, controlling, and predicting quality and productivity in biomanufacturing. The use of proven software with guided PCA and PLS model creation means you don’t need to be a data scientist to explore and analyze your data.
- Software Simplifies Compliance with 21 CFR Part 11 and EudraLex Good Manufacturing Practice Volume 4 Annex 11
How software can simplify 21 CFR Part 11 and EudraLex Volume 4 Annex 11 compliance and provide additional benefits such as access control, audit trails and easier search and retrieval.
- Data Sheet: Bio4C ProcessPad™ Software
Bio4C ProcessPad™ software is a data collection, visualization, and analytics platform that enables bioprocess monitoring, lifecycle management, reporting, investigations, and continued process verification (CPV).
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?