464465
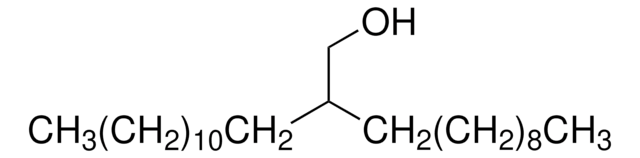
2-Butyl-1-octanol
95%
Sinónimos:
2-Butyloctanol, 2-Butyloctyl alcohol, 5-(Hydroxymethyl)undecane, Butyloctanol, Guerbet dodecanol
About This Item
Productos recomendados
assay
95%
bp
145-149 °C (lit.)
density
0.833 g/mL at 25 °C (lit.)
SMILES string
CCCCCCC(CO)CCCC
InChI
1S/C12H26O/c1-3-5-7-8-10-12(11-13)9-6-4-2/h12-13H,3-11H2,1-2H3
InChI key
XMVBHZBLHNOQON-UHFFFAOYSA-N
General description
Application
- 2-butyl-1-octyl-methacrylate (BOMA)
- 3,5,5-trimethyl-1-hexyl methacrylate (TMHMA)
- hydrophobic polyesters in miniemulsion in the presence of large amounts of water
It has also been used as an extraction solvent in extractive fed-batch experiments.
signalword
Warning
hcodes
pcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
251.6 °F - Non-equilibrium method
flash_point_c
122 °C - Non-equilibrium method
ppe
Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico