BCR311
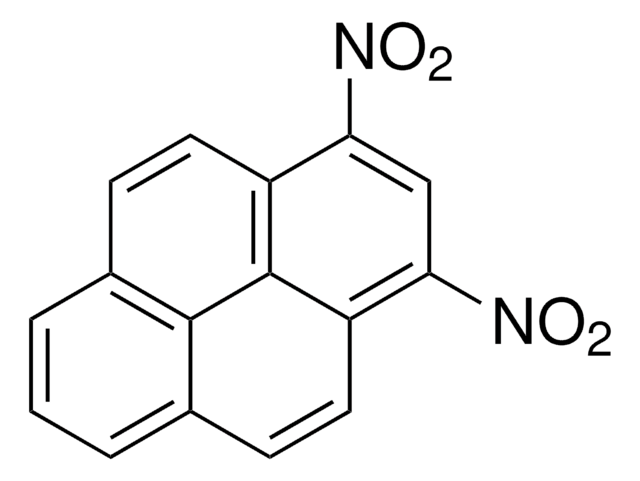
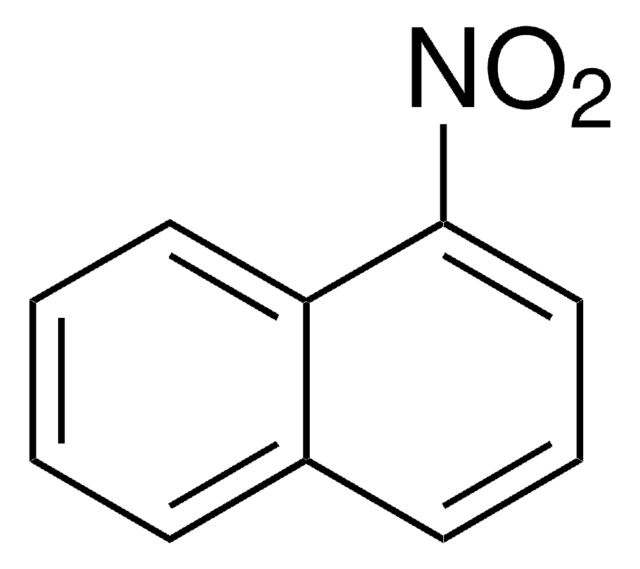
6-Nitrobenzo[a]pyrene
BCR®, certified reference material
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H11NO2
CAS Number:
Molecular Weight:
297.31
Beilstein:
2472924
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
certified reference material
Agency
BCR®
manufacturer/tradename
JRC
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
format
neat
storage temp.
2-8°C
SMILES string
[O-][N+](=O)c1c2ccccc2c3ccc4cccc5ccc1c3c45
InChI
1S/C20H11NO2/c22-21(23)20-16-7-2-1-6-14(16)15-10-8-12-4-3-5-13-9-11-17(20)19(15)18(12)13/h1-11H
InChI key
NMMAFYSZGOFZCM-UHFFFAOYSA-N
General description
6-Nitrobenzo[a]pyrene belongs to the class of nitrated-polycyclic aromatic hydrocarbons, found to be persistent in the environment. It is produced from direct sources such as diesel, gasoline exhaust and by the gas-phase reactions of polycyclic aromatic hydrocarbons (PAHs) with oxides of nitrogen.
Analysis Note
For more information please see:
BCR311
BCR311
Legal Information
BCR is a registered trademark of European Commission
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Effect of nitro substitution on the light-mediated mutagenicity of polycyclic aromatic hydrocarbons in Salmonella typhimurium TA98.
G L White et al.
Mutation research, 144(1), 1-7 (1985-09-01)
Kazuichi Hayakawa et al.
International journal of environmental research and public health, 17(2) (2020-01-16)
Total suspended particulate matter (TSP) was collected during the summer and winter in five cities in China (Shenyang, Beijing, and Shanghai), Russia (Vladivostok), and Korea (Busan) from 1997 to 2014. Nine polycyclic aromatic hydrocarbons (PAHs) with four to six rings
C Raha et al.
Journal of toxicology and environmental health, 19(1), 55-64 (1986-01-01)
Nitropolycyclic aromatic hydrocarbons (nitroarenes), including 6-nitrobenzo[a]pyrene (6-NBap), occur in our environment and are mutagenic in bacterial mutagenesis assays. The mutagenicity of 6-NBaP is enhanced when rat liver S9 is added. To investigate the cause of this increased activity, the metabolism
G Löfroth et al.
Carcinogenesis, 5(7), 925-930 (1984-07-01)
Several nitroarenes derived from benzo[a]pyrene and perylene and the parent hydrocarbons have been assayed for mutagenicity in the Salmonella microsome test and for affinity for the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-receptor protein in rat liver cytosol. 1- and 3-nitrobenzo[a]pyrene are mutagenic in the
B S Hass et al.
Carcinogenesis, 7(4), 681-684 (1986-04-01)
Previous studies have shown that 1- and 3-nitrobenzo[a]pyrene (NBaP) were mutagenic in the Salmonella reversion assay without exogenous activation and that 1-, 3- and 6-NBaP were mutagenic in the presence of hepatocytes or liver homogenate (S9). In the present study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service