389439
1-Pyrenemethanol
98%
Synonyme(s) :
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C17H12O
Numéro CAS:
Poids moléculaire :
232.28
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Essai
98%
Forme
solid
Pf
123-126 °C (lit.)
Groupe fonctionnel
hydroxyl
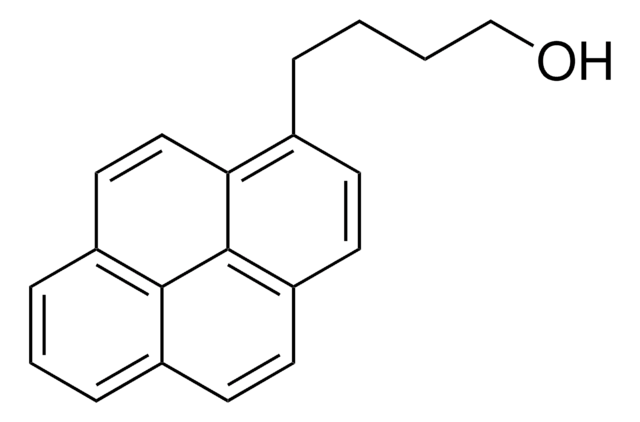
Chaîne SMILES
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
Clé InChI
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Application
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
C D Sherman et al.
Carcinogenesis, 16(10), 2499-2506 (1995-10-01)
The promotional effect of phenobarbital and 1-hydroxymethyl-pyren on enzyme altered lesions in the rat liver were quantified within the framework of two separate multipath/multistage models. The experiment analyzed followed an initiation-promotion protocol in which female Wistar rats were initiated with
Ronny Kollock et al.
Biochemical pharmacology, 75(2), 527-537 (2007-10-09)
Alkylated polycyclic aromatic hydrocarbons can be metabolically activated via benzylic hydroxylation and sulphation to electrophilically reactive esters. However, we previously found that the predominant biotransformation route for the hepatocarcinogen 1-hydroxymethylpyrene (1-HMP) in the rat in vivo is the oxidation of
H Glatt et al.
Environmental health perspectives, 88, 43-48 (1990-08-01)
Methylated polycyclic aromatic hydrocarbons are common in the human environment. Many of them are stronger carcinogens than their purely aromatic congeners. They may be metabolized to benzylic alcohols. We report here on biochemical and toxicological characteristics of 1-hydroxymethylpyrene (HMP), a
H Glatt et al.
Mutation research, 324(3), 111-114 (1994-07-01)
Previous studies demonstrated that the ion composition of the exposure medium may strongly influence the mutagenicity of many compounds in the liquid preincubation modification of the reversion assay with his- Salmonella typhimurium strains. Similar influences were now observed in the
Bernhard H Monien et al.
Toxicology, 262(1), 80-85 (2009-06-02)
1-Methylpyrene (1-MP), an abundant alkylated polycyclic aromatic hydrocarbon, is activated by side-chain hydroxylation to 1-hydroxymethylpyrene (1-HMP) and subsequent sulfo-conjugation to electrophilic 1-sulfooxymethylpyrene (1-SMP). In rats, this activation mainly occurs in liver. 1-SMP may react with hepatic DNA or be exported
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique







![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)