All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O2
CAS Number:
Molecular Weight:
112.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.474 (lit.)
bp
76-78 °C/15 mmHg (lit.)
density
1.13 g/mL at 25 °C (lit.)
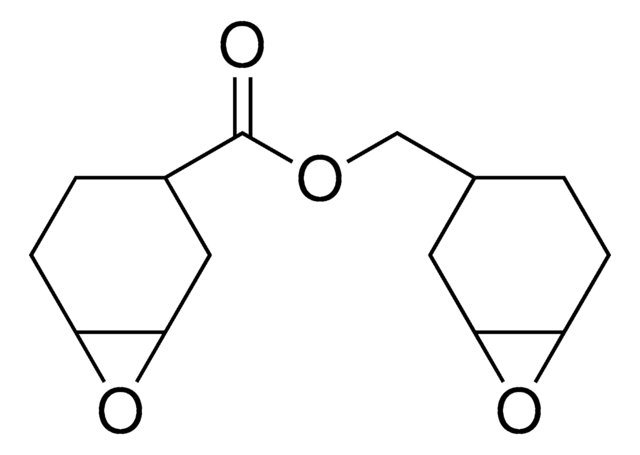
SMILES string
O=C1CCCC2OC12
InChI
1S/C6H8O2/c7-4-2-1-3-5-6(4)8-5/h5-6H,1-3H2
InChI key
QKOHEJBTNOEACF-UHFFFAOYSA-N
General description
7-Oxabicyclo[4.1.0]heptan-2-one is one of the products formed during oxidation of cyclohexene by dendritic complexes. It has been reported as anticapsin analog.
Application
7-Oxabicyclo[4.1.0]heptan-2-one was employed as substrate to investigate the substrate specificity of purified recombinant NADPH-dependent 3-quinuclidinone reductases from Microbacterium luteolum JCM 9174 for the reductive reaction of ketones.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kentaro Isotani et al.
Applied and environmental microbiology, 79(4), 1378-1384 (2012-12-25)
We used the resting-cell reaction to screen approximately 200 microorganisms for biocatalysts which reduce 3-quinuclidinone to optically pure (R)-(-)-3-quinuclidinol. Microbacterium luteolum JCM 9174 was selected as the most suitable organism. The genes encoding the protein products that reduced 3-quinuclidinone were
Oxidation of cyclohexene by dendritic PAMAMSA-Mn (II) complexes.
Yang Z-W, et al.
J. Mol. Catal. A: Chem., 213(2), 169-176 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![7-Oxabicyclo[2.2.1]heptane 98%](/deepweb/assets/sigmaaldrich/product/structures/377/935/931d29d9-08c9-492a-b42e-3f8f5a20f595/640/931d29d9-08c9-492a-b42e-3f8f5a20f595.png)