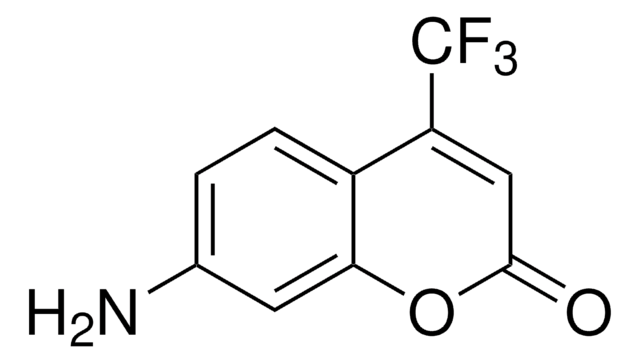
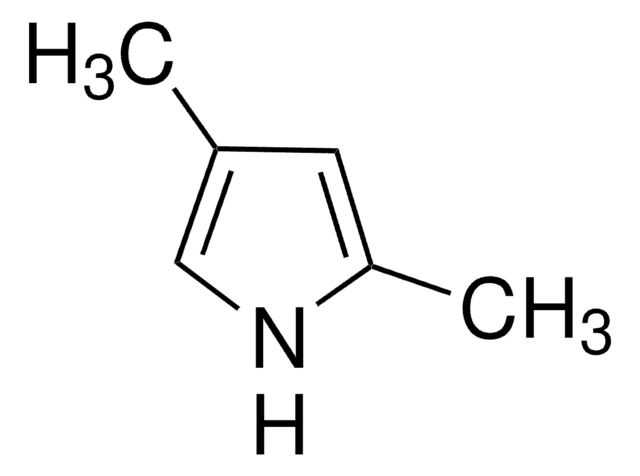
790389
Difluoro{2-[1-(3,5-dimethyl-2H-pyrrol-2-ylidene-N)ethyl]-3,5-dimethyl-1H-pyrrolato-N}boron
99% (HPLC)
Sinónimos:
1,3,5,7,8-Pentamethyl-4,4-difluorro-4-bora-3a,4a-diaza-s-indacene, 2-[1-(3,5-Dimethyl-2H-pyrrol-2-ylidene)ethyl]-3,,5-dimethyl-1H-pyrrole, boron complex, 4,4-Difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene, BODIPY 493/503, PM 546, PMPBF2, Pyrromethene 546, [[2-[1-(3,5-Dimethyl-2H-pyrrol-2-ylidene)ethyl]-3,5-dimethyl-1H-pyrrolato-N1,N2]difluoro]boron
About This Item
Productos recomendados
assay
99% (HPLC)
form
powder
mp
262-266 °C
fluorescence
λex 493 nm; λem 504 nm in methanol
SMILES string
CC(C1=C(C)C=C(C)N1B(F)2F)=C3[N]2=C(C)C=C3C
InChI
1S/C14H17BF2N2/c1-8-6-10(3)18-13(8)12(5)14-9(2)7-11(4)19(14)15(18,16)17/h6-7H,1-5H3
InChI key
DRJHPEGNOPSARR-UHFFFAOYSA-N
General description
Application
Also used for solid-state dye laser devices and organic solar cells.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![Difluoro{2-[(3,5-dimethyl-2H-pyrrol-2-ylidene-N)methyl]-3,5-dimethyl-1H-pyrrolato-N}boron 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/518/861/c19c64be-654e-472e-a069-30ffccb1a8cd/640/c19c64be-654e-472e-a069-30ffccb1a8cd.png)


![Difluoro(4-(1,1-dimethylethyl)-2-{1-[4-(1,1-dimethylethyl)-3,5-dimethyl-2H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrolato-N)boron 98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/879/8046aafd-78ca-4fd8-92dc-801de0b6cc53/640/8046aafd-78ca-4fd8-92dc-801de0b6cc53.png)