778583
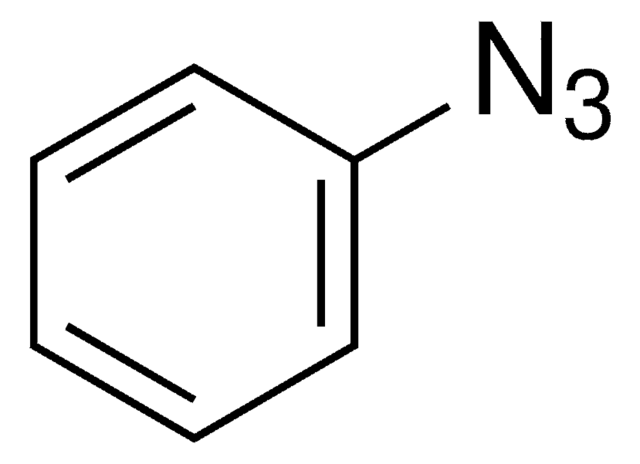
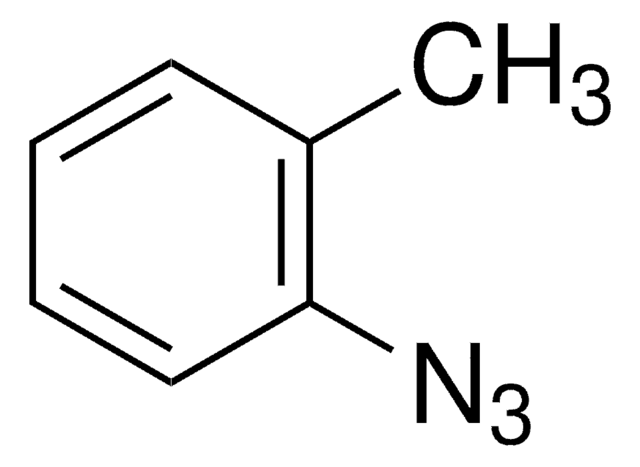
Azidobenzene solution
~0.5 M in 2-methyltetrahydrofuran, ≥95.0% (HPLC)
Sinónimos:
Phenyl azide solution
About This Item
Productos recomendados
assay
≥95.0% (HPLC)
form
liquid
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
~0.5 M in 2-methyltetrahydrofuran
impurities
≤2.0% water
greener alternative category
storage temp.
−20°C
SMILES string
[N-]=[N+]=Nc1ccccc1
InChI
1S/C6H5N3/c7-9-8-6-4-2-1-3-5-6/h1-5H
InChI key
CTRLRINCMYICJO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT RE 2
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
10.4 °F
flash_point_c
-12 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico