SuFEx: Sulfonyl Fluorides that Participate in the Next Click Reaction
Featuring ESF Reagent for the Easy Installation of SO2F
Introduction
Sulfur (VI) fluoride exchange (SuFEx) has been identified as the next click chemistry reaction owing to the balanced properties of fluoride bonding to hydrogen and sulfur (VI). In collaboration with K. Barry Sharpless, we now offer a variety of sulfonyl fluoride reagents that undergo a newly defined click chemistry reaction.1 SuFEx building blocks provide “connective” chemistry that will find wide utility in applications such as chemical synthesis, materials science, chemical biology, and drug development.1-4


1. Resistance to reduction:In contrast to other halides, sulfonyl fluoride cleavage is heterolytic and thus resistant to reduction.1

2. Thermodynamic stability:Sulfonyl fluorides are stable to thermolysis as well as nucleophilic substitution1 and have notably exhibited inert reactivity in refluxing aniline.2

3. Exclusive reaction at sulfur:Sulfonyl fluorides react fast and, compared to sulfonyl chlorides, chemoselectively produce only sulfonylation products.1

4. Special nature of the fluoride-proton interaction:Stabilization of the fluoride ion in water affords chemistry in aqueous environments.1

Among our available sulfonyl fluoride building blocks, we offer ethenesulfonyl fluoride (Product No. 746959), ESF, a Michael acceptor for N, O, S, and C nucleophiles. Therefore, the SO2F functional group can be readily incorporated into molecules for drug discovery and chemical biology applications. The generated sulfonyl fluoride is extremely stable but readily reacts with nucleophiles under the appropriate conditions for further functionalization or biochemical use.1-2

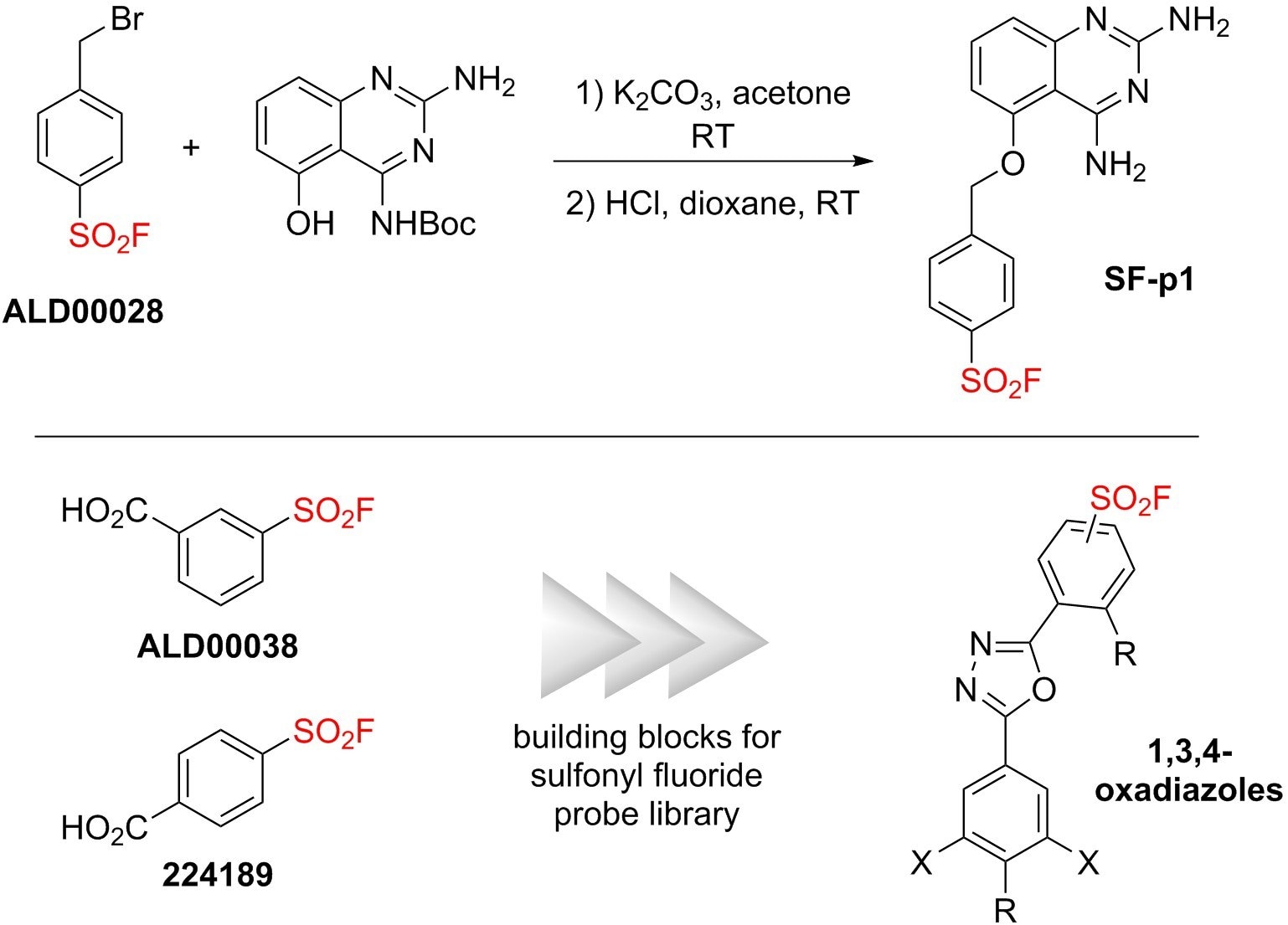
Other SuFEx building blocks have been used to introduce SO2F handles for applications in chemical biology, such as on the probe used by Jones and co-workers (SF-p1)5 and in the library generated by Kelly and his colleagues (1,3,4-oxadiazoles).6
Chemical Biology and Drug Discovery
When integrated on known bioactive molecules, the biocompatibility and reactivity of sulfonyl fluorides provide powerful tools for chemical biologists and drug developers.4 The SO2F group covalently modifies many protein residues but in a context-specific manner: serine, threonine, tyrosine, lysine, cysteine, and histidine.4 As such, sulfonyl fluorides can be uniquely enlisted in chemical probes, covalent inhibitors, or various stages of your translational workflow, including target validation and lead optimization/late-stage functionalization
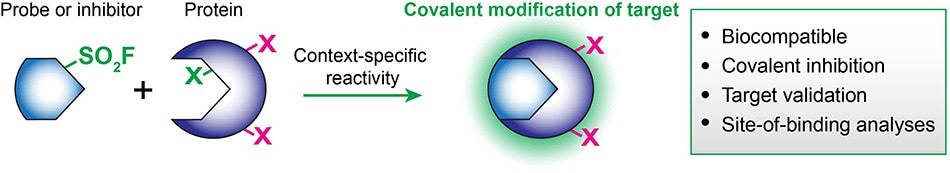
Some recent examples of the functionalization of previous inhibitors and enzyme stabilizers with SO2F resulted in the chemoselective modification of proteins of interest: DcpS on Tyr,5 transthyretin on Lys,6 and polyisoprenylated methylated protein methyl esterase on Ser.7 Other well-known sulfonyl fluoride inhibitors include PMSF (P7626, 78830, 93482), AEMSF (76307, 15633, A8456) and FSBA (F9128).

To learn more about the biological applications of SuFEx, check out our webinar with Lyn Jones of Pfizer! "Sulfonyl Fluorides in Chemical Biology and Drug Development"
Explore other products in our catalog that have been established in collaboration with Professor Sharpless at his Professor Product Portal.
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?