64306
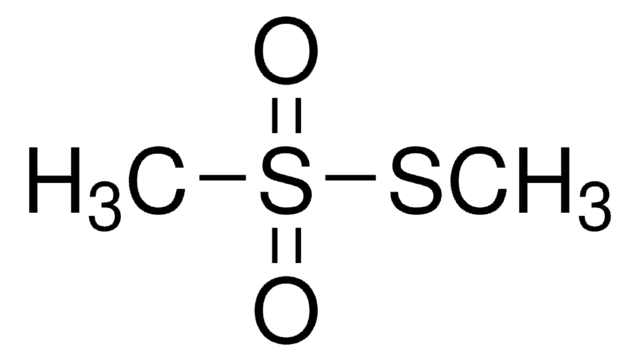
S-Methyl methanethiosulfonate
purum, ≥98.0% (GC)
Sinonimo/i:
S-Methyl thiomethanesulfonate, MMTS
About This Item
Prodotti consigliati
Grado
purum
Livello qualitativo
Saggio
≥98.0% (GC)
Indice di rifrazione
n20/D 1.513 (lit.)
n20/D 1.513
P. eboll.
69-71 °C/0.4 mmHg (lit.)
Solubilità
chloroform: 750mg + 5 ml Chloroform mg/mL, colorless to light greenish-yellow
Densità
1.337 g/mL at 20 °C
1.337 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
CSS(C)(=O)=O
InChI
1S/C2H6O2S2/c1-5-6(2,3)4/h1-2H3
XYONNSVDNIRXKZ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Modification of Thiol Enzymes: S-methyl methanethiosulfonate (MMTS) offers a unique method for the modification of thiol enzymes and redox-regulated proteins, providing potential applications in biochemical research focused on enzyme regulation and redox biology (Makarov et al., 2019).
- Sensor Development for Protease Activity: S-methyl methanethiosulfonate is used as a blocking reagent on the structural transitions of papain-like cysteine proteases, which supports its utility in sensor development, allowing for the detection and analysis of protease activity in various biological processes (Markovic et al., 2023).
- Agricultural Pathogen Control: Research evaluating S-methyl methanethiosulfonate as a late blight inhibitor highlights its potential as a broad-range toxin against plant pathogens, suggesting applications in agriculture for the management of crop diseases (Joller et al., 2020).
Avvertenza
Altre note
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
188.6 °F - closed cup
Punto d’infiammabilità (°C)
87 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.