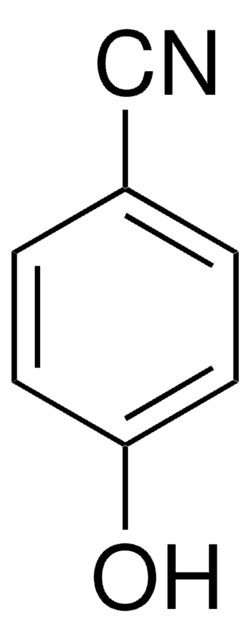
345806
2,4,6-Trifluorophenol
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
F3C6H2OH
Numero CAS:
Peso molecolare:
148.08
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Stato
solid
Punto di fusione
49-51 °C (lit.)
Stringa SMILE
Oc1c(F)cc(F)cc1F
InChI
1S/C6H3F3O/c7-3-1-4(8)6(10)5(9)2-3/h1-2,10H
QQFWMPUXPLBWTG-UHFFFAOYSA-N
Descrizione generale
2,4,6-Trifluorophenol is a halogen-substituted phenol. Peroxidase oxidation of 2,4,6-trifluorophenol has been reported. Association of phenol and 2,4,6-trifluorophenol has been investigated by 1H- and 19F-NMR spectroscopy.
Applicazioni
2,4,6-Trifluorophenol may be used in an indirect enzyme-linked immunosorbent assay (ELISA) for the biological monitoring of 2,4,6-trichlorophenol.
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Revisiting the peroxidase oxidation of 2,4,6-trihalophenols: ESR detection of radical intermediates.
Bradley E Sturgeon et al.
Chemical research in toxicology, 24(11), 1862-1868 (2011-09-29)
The peroxidase oxidation of 2,4,6-trichlorophenol (TCP) has been clearly shown to result in 2,6-dichloro-1,4-benzoquinone (DCQ). DCQ is a 2-electron oxidation product of TCP that has undergone para dechlorination. Many peroxidases show similar oxidation of the substrate, TCP, to yield the
Daniela Smejkalová et al.
Environmental science & technology, 43(14), 5377-5382 (2009-08-28)
1H- and 19F-NMR measurements of spin-lattice (T1) and spin-spin (T2) relaxationtimes and diffusion ordered spectroscopy (DOSY) were applied to investigate the association of nonsubstituted (phenol (P)) and halogen-substituted (2,4-dichlorophenol (DCP); 2,4,6-trichlorophenol (TCP), and 2,4,6-trifluorophenol (TFP) phenols with a dissolved humic
Roger Galve et al.
Analytical chemistry, 74(2), 468-478 (2002-01-29)
Trichlorophenols (TCP) eliminated by the urine can be considered as potential biomarkers of exposure of many chemicals (chlorophenols, chlorophenoxy acid herbicides, prochloraz, lindane, hexachlorobenzene, etc). High-throughput screening methods are necessary to carry out efficient monitoring programs that may help to
Erin M Kollitz et al.
Toxicological sciences : an official journal of the Society of Toxicology, 163(1), 226-239 (2018-02-07)
Brominated phenolic compounds (BPCs) are found in the environment, and in human and wildlife tissues, and some are considered to have endocrine disrupting activities. The goal of this study was to determine how structural differences of 3 BPC classes impact
Marie A Colucci et al.
Journal of medicinal chemistry, 50(23), 5780-5789 (2007-10-20)
NAD(P)H:quinone oxidoreductase 1 is a proposed target in pancreatic cancer. We describe the synthesis of a series of indolequinones, based on the 5- and 6-methoxy-1,2-dimethylindole-4,7-dione chromophores with a range of phenolic leaving groups at the (indol-3-yl)methyl position. The ability of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.